Abstract
The series of adducts of magnesium beta-diketonates with diamimes namely Mg(thd)2(tmeda) 1 and four new complexes Mg(thd)2(tmpda) 2, Mg(thd)2(pda) 3, Mg(ptac)2(tmeda) 4, Mg(tfac)2(tmeda) 5 [beta-diketonates = R1C(O)CHC(O)R2: thd (R1 = R2 = tBu), ptac (R1 = tBu, R2 = CF3), tfac (R1 = Me, R2 = CF3); diamines = R2N(CH2) n NR2: tmeda (n = 2, R = Me), tmpda (n = 3, R = Me), pda (n = 3, R = H)] were synthesized in order to investigate the influence of both ligand types on the thermochemical properties of these compounds. The thermal behavior of the complexes in the condensed phase was investigated by thermogravimetry and differential scanning calorimetry; the thermodynamic parameters of phase transitions were determined. The saturated vapor pressure of solid complexes Mg(thd)2(tmeda) and Mg(tfac)2(tmeda) were measured by the transmission method giving the enthalpies and entropies of sublimation processes. The reliable thermodynamic data for compound Mg(thd)2(tmeda) were obtained with the assistance of X-ray diffraction and the static method of saturated and unsaturated vapor pressure measurements.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Metal-organic chemical vapor deposition (MOCVD) of magnesium oxide have been intensively developed for the last few decades due to wide use of MgO thin films as buffer, dielectric, optical, protective, or emissive layers in microelectronics [1–4].
The choice of volatile meta-organic compounds (precursors) plays the key role in successful realization of MOCVD processes because the experimental conditions and film characteristics are determined by thermochemical properties of volatile precursors. A great number of organometallic and coordination compounds containing (O,C,N)-ligands of various organic classes (alkyls, alkoxides, beta-diketonates, beta-ketoiminates, carbamates, cyclopentadienyls, diamines, etc.) have been used as MgO MOCVD precursors [1–3, 5–14].
Among them, the adducts of magnesium beta-diketonates with diamines Mg(beta-diketonato)2(diamine) are one of the most perspective class due to their synthetic accessibility, and good volatility and stability in the condensed and gas phases [3, 13, 15]. Beside that, the thermal properties of these complexes potentially could be varied in the wide range by simple modification of two ligand types: beta-diketonates and diamines. The effects of ethylenediamine derivatives on the structures and thermal behaviors of the Mg(thd)2(diamine) and Mg(hfac)2(diamine) type complexes were studied by Hatanpää [15] and Marks [3], respectively. So, the idea of the present work was the transition from ethylenediamine derivatives to propylenediamine ones on the “Mg(thd)2” core and transition to beta-diketonates containing CF3- and unfluorinated substitutes on the “Mg(diamine)2” core. It should be noted that fluorinated complexes also could be used as MOCVD precursors to form the oxide films at introduction of water vapors to the deposition zone [3]. Moreover, the complexes with fluorinated beta-diketonates are perspective as the universal precursors for deposition of oxide or fluoride phases depending on the experimental conditions [16].
Herein, the aim of our work was the investigation of ligand influence on the thermochemical properties of Mg(beta-diketonato)2(diamine) compounds. Mg(thd)2(tmeda) 1 [13, 15] was the model complex, while both ligand types were varied yielding four new complexes (Fig. 1; the abbreviation are listed in Table 1). The diamine change was illustrated by Mg(thd)2(tmpda) 2 and Mg(thd)2(pda) 3 compounds; beta-diketonate change was presented by Mg(ptac)2(tmeda) 4 and Mg(tfac)2(tmeda) 5 complexes (Fig. 1). Thermal properties of the obtained compounds in condensed phase were studied by thermogravimetry and differential scanning calorimetry. The thermochemical properties of complex 1 were published previously [17]; in our work, these data were clarified with the help of X-ray diffraction analysis and two independent methods of saturated vapor pressure measurements: transpiration and static. Also, the saturated vapor pressure on the solid complex 5 was investigated by the transpiration method as first the example of such precise investigations of the magnesium complexes with fluorinated ligands.
Experimental
All chemicals were commercially available products of reagent grade and used without further purification. Magnesium beta-diketonates (Mg(thd)2(H2O)2, Mg(ptac)2, and Mg(tfac)2(H2O)2·1.5H2O) were synthesized by previously reported procedure [18]. The CHN analysis of compounds 1–3 was performed using Model CARLO-ERBA-11008 elemental analyzer; the CHNF analysis of compounds 4 and 5 was carried out in Vorozhtsov Novosibirsk Institute of Organic Chemistry SB RAS [19, 20]. The error of determination for both methods did not exceed 0.5 %. 1H NMR spectra were recorded using Bruker Avance-500 instrument (500.13 MHz) for solutions of individual compounds in CDCl3. The IR absorption spectra within the range 4,000–400 cm−1 were recorded in KBr tablets for complexes 2–5 and in a thin layer between KBr glass plates for the liquid free diamines. The attribution of absorption bands was performed using literature data [21, 22].
Mg(thd)2(tmeda) (1) was obtained as described in [13] with purification by recrystallization from toluene. Yield 80 %. Anal. Calcd. for C28H54O4N2Mg: C, 66.3; H, 10.7; N, 5.5. Found: C, 66.9; H, 11.1; N, 5.3.
Mg(thd)2(tmpda) (2), Mg(thd)2(pda) (3), Mg(ptac)2(tmeda) (4), and Mg(tfac)2(tmeda) (5) were synthesized by a modification of literature method [15]. Magnesium beta-diketonate (1–1.5 mmol) and hexane (10–15 mL) were placed in Wurtz flask. Nitrogen flow was passed through the mixture under stirring at room temperature for 0.5 h, and then the excess of diamine was added. The main part of white solid dissolved immediately, but the reaction mixture was stirred under nitrogen flow for additional 1 h. The resulted solution was filtered, and the solvent and unreacted diamine were removed in vacuo (for compounds 2 and 3) or under nitrogen flow (for compounds 4 and 5). The crude products were purified by recrystallization from hexane. IR and 1H NMR data are summarized in Table 2.
Mg(thd)2(tmpda) (2). From 0.66 g (1.55 mmol) Mg(thd)2(H2O)2 and 0.52 g (0.77 mL, 5.10 mmol) tmpda 0.60 g (1.15 mmol), compound 2 was obtained. Yield 74 %. Colorless crystals. Anal. Calcd. (at.%) for C29H56O4N2Mg: C, 66.9; H, 10.8; N, 5.4. Found: C, 67.1; H, 10.8; N, 5.4.
Mg(thd)2(pda) (3). From 0.45 g (1.05 mmol) Mg(thd)2(H2O)2 and 0.16 g (0.18 mL, 2.10 mmol) pda 0.40 g (0.86 mmol), compound 3 was obtained. Yield 82 %. Colorless crystals. Anal. Calcd. (at.%) for C25H48O4N2Mg: C, 64.6; H, 10.4; N, 6.0. Found: C, 64.3; H, 10.4; N, 6.1.
Mg(ptac)2(tmeda) (4). From 0.40 g (1.00 mmol) Mg(ptac)2 and 0.14 g (0.18 mL, 1.20 mmol) tmeda 0.46 g (0.87 mmol), complex 4 was obtained. Yield 87 %. Colorless crystals. Anal. Calcd. (at.%) for C22H36F6O4N2Mg: C, 49.8; H, 6.8; N, 5.3; F, 21.5. Found: C, 49.6; H, 6.6; N, 5.0; F, 21.5.
Mg(tfac)2(tmeda) (5). From 0.40 g (0.97 mmol) Mg(tfac)2(H2O)2·1.5 H2O and 0.14 g (0.18 mL, 1.20 mmol) tmeda 0.36 g (0.80 mmol), complex 5 was obtained. Yield 80 %. Colorless crystals.Anal. Calcd. (at.%) for C16H24F6O4N2Mg: C, 43.0; H, 5.4; N, 6.3; F, 25.5. Found: C, 43.0; H, 5.4; N, 6.2; F, 25.6.
TG investigation of compounds 1–5 was carried out using thermoanalizator Netzsch STA 409 PC/PG (He, 20 mL min−1, 10 K min−1, 298–873 K, sample mass 11 mg). Calorimetric measurements were performed using a Setaram DSC 111 (0.5–1.0 K min−1; sample mass 13–25 mg) to define thermodynamic characteristics of phase transition (T tr, Δtr H). The errors in the heat effect measurements estimated from calibration experiments (C6H5COOH, In) were less than 1.5 %. During measurements, the investigated substances were contained in evacuated glass ampoule. Three-five calorimetric experiments were carried out for each compound. The obtained thermodynamic characteristics are presented in Table 2.
The temperature dependencies of saturated vapor pressure for complexes 1 and 5 were measured by transmission method. The used setup details of measurements and calculations are described in [23, 24]. The experiments were carried out in atmosphere of helium as a dry inert gas carrier in temperature ranges 373–483 K and 333–363 K for complexes 1 and 5, respectively. The helium flow rates were ranged from 0.9 to 1.8 l h−1. Total uncertainty of this method was not above 5 % with accuracy in the maintenance of temperature 0.5 K, the uncertainty in the measurement of flow rate 2 %, and in mass registration 5 × 10−4 g.
The static method with membrane zero manometer was employed to measure saturated and unsaturated vapor pressure of solid complex 1 in temperature range 422–472 K. The detailed experimental procedures and description of setup are presented in [25]. The pressure and temperature measurement uncertainties were less than 40 Pa and 0.5 K, respectively. The processing data were carried out by least squares method with the criterion function based on the principle of maximal likelihood [26].
X-ray diffraction (XRD) analysis of polycrystals was performed on Shimadzu XRD-7000 diffractometer (Cu K-alpha radiation, Ni-filter, 5–60° 2θ range, 0.03° 2θ step size, 1 s per step). Polycrystalline samples were slightly ground in an agate mortar, and the resulting powder was deposited on the polished side of a standard quartz sample holder cover with vaseline oil. To obtain the diffraction pattern for the structure of complex 1 after the phase transition at 420.7 K, we used the following procedure. The portion of complex 1 was placed into quartz ampoule, brought to pressure 1.333 Pa, sealed and then heated tube furnace at 433 K for 2.5 h. Then, it was rapidly cooled by dipping into the liquid nitrogen and opened. The powder of complex 1 was prepared to XRD experiment as described below. The theoretical diffraction patterns of complexes Mg(thd)2(tmeda) and [Mg(thd)2]2 were calculated from the single-crystal XRD data available in the Cambridge Structural Database (CSD codes GUHPUU and XOJTEV, respectively).
Results and discussion
Synthesis of the complexes and their thermal properties in the condensed phases
The model Mg(thd)2(tmeda) 1 complex was synthesized by previously reported one-pot synthesis in water–ethanol solution [13], while to obtain the other compounds 2–5, it was necessary to separate the corresponding magnesium beta-diketonate like in [15]. Opposite to the work [3] reported the successful one-pot synthesis for Mg(hfac)2(diamine) complexes, all attempts to obtain complex 4 with ptac-ligand by this method led to the separation of (H2tmeda)[Mg(ptac)3]2 6 [27]. This result could be explained in terms of the low solubility of complex 6, while the analog phases for hfac are more label and, so, the reorganization of the structures could occur forming the desired adducts. This assumption may be confirmed by the preparing compound contained Hteeda+ cation and Mg(hfac)3-anion and also by relatively low yields (40–65 %) of hfac adducts with other diamines described in the work [3].
It should also be mentioned that according to our results and the previous data [3, 15], all tmeda-adducts are stable while keeping at air, whereas complexes contained propylenediamine derivatives (compounds 2 and 3) decompose giving magnesium beta-diketonate during the storage. The data of elemental CHN analysis have shown that 50 % decreasing of the nitrogen content for complex 3 was achieved after 1 month storage, while that for complex 2 was only 1 week.
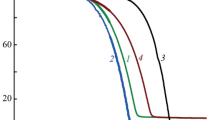
The mass loss curves for complexes 1–5 are presented in Fig. 2. All complexes transfer into the gas phase leaving less than 2 % residue. The DTG curves for complexes 4 and 5 exhibit only one peak indicating that the vaporization processes are clear. The two peaks on DTG curves for complex 1–3 testify the fact that their vaporizations are accompanied by the decomposition processes (i.e., the dissociation to the magnesium beta-diketonate and diamine). This fact have been already noted for the Mg(thd)2(diamine) [15] generating the interest to the detailed investigation of the stability of Mg(thd)2(tmeda) as the most attractive to MOCVD [28]. It was shown that at the “right” conditions, the sublimation without decomposition could be achieved [15, 17]. Comparison of the stability of adducts with ethylenediamine derivatives and that for propylenediamine ones has shown that the decomposition of the latter complexes is more intensive (the steps on the mass loss curves, Fig. 2). In our opinion, this fact is concerned with stress accumulation in the appropriate chelate cycles: according to single-crystal XRD data, the NMgN chelate angle significantly increases from 79.51(8)° (1) to 85.18(5)° (3) and 89.46(10)°(2) (some structural information are presented in Supplementary material). The same order was observed for the “life times” of complexes at air and for clarity of the steps on the mass loss curves (Fig. 2) and their derivatives. The strong decomposition of complexes with pda and tmpda ligands does not allow us to make of any conclusions about the relative volatility of investigated complexes with “Mg(thd)2” core.
Summing the presented results with data of Hatanpää [15] and Marks [3], we could conclude that the “slight” change in the diamine ligand (i.e., the replacements of substitutes on the nitrogen atom from H to Me, Et or their combination) does not lead to principal changes of Mg(beta-diketonato)2(diamine) volatility and stability. The changing of the diamine chelate cycle size (ethylenediamine → propylenediamine derivatives, the present work) and transition to poliamines (ethylenediamine → dimethylethylenetriamine, etc., [15] ) result in the decrease of adduct stability.
Comparing thermal behavior of complexes 1, 4, and 5, we could conclude that adducts of fluorinated beta-diketonates are more stable under the heating probably due to the more strong attraction between diamine ligand and magnesium beta-diketonate core as a result of the formation of larger positive charge on magnesium atom under the influence of electron-acceptor group CF3. In general, the thermogravimetry data do not allow us to construct the qualitative sequence of volatility for complexes 1, 4, and 5.
The results of DSC investigation for complexes are presented in Table 3. As it was expected, complex 5 with fluorinated beta-diketonates melts at lower temperature than compounds with thd-ligand. The melting point of complex 2 containing methyl substitutes on nitrogen atoms is higher than that of its hydrogen-substituted analog (complex 3); however, the enthalpies of melting processes are close. Concerning analog compounds with ethylenediamine derivatives, Mg(thd)2(en) melted at 425 K [15], whereas some different data were published for Mg(thd)2(tmeda) 1: 373 K [13], 418 K [17], and 425 K [15]. The DCS investigation has shown that complex 1 has two endothermic peaks at 420.7 and 489.0 K (Fig. 3). Heating sample was stopped after first peak, and we have convinced visually that the substance was not melted. Therefore, this peak corresponds to the solid–solid phase transition. This transition is reversible because it also occurs during cooling of the sample. A detailed XRD study has been carried out to clarify the nature of this phase transition (Fig. 4). The diffraction pattern of complex 1 powder corresponds to the theoretical one (Fig. 41a and 1c curves). To obtain the diffraction pattern for the structure of complex 1 after the phase transition studied, the compound was kept at 433 K for several hours, and then the sample was rapidly cooled in liquid nitrogen. The significant changes in the diffraction pattern are observed (Fig. 41a and 1b curves). It could be seen from Fig. 4 that the decomposition of complex 1 with formation of [Mg(thd)2]2 does not take place in this case. Herein, the phase transition at 420.7 K is structural. So, complex 1 melts only at 489.0 K. The thermodynamic parameters of this process are rather low. This fact is connected with structural reconstruction during the endothermic phase transition before melting. In general, complexes Mg(thd)2(tmeda) 1 and Mg(thd)2(en) also satisfy the observation mentioned above that Mg(thd)2 adducts with N,N,N′,N′-tetramethyldiamines melt at higher temperatures than their analogs with primary diamines.
Vapor pressure investigation
Complexes 1 and 5 were chosen for saturated vapor pressure measurements as ones of the most volatile compounds according to TG data. The transpiration method was employed for this study as the most close to the MOCVD experimental conditions in the flow-type reactors. As the saturated vapor pressure data for complex 1 were published earlier, we appealed to the static method as the second independent investigation technique. The experimental data are shown in Fig. 5. The p(T) equations, enthalpies, and entropies of sublimation processes are presented in Table 4.
Temperature dependences of saturated vapor pressure of complexes 1—Mg(thd)2(tmeda) [transmission method (a), static method (b), data [17] (c)] and 5—Mg(tfac)2(tmeda); p 0 = 101,325 Pa
For complex 1, the measurements were carried out by transmission method in the temperature range up to structural phase transition (373–413 K). The mass of compound left the source, and mass of compound condensed in the receiver differed insignificantly during up to 20 h indicating the absence of any noticeable decomposition processes. The results of elemental analyses of sublimates also did not differ from pure complex 1. So, the temperature range 373–413 K could be used as evaporator temperature in the CVD apparatus of flow type (used the carrier gas).
Saturated vapor pressure was measured by the static method with membrane zero manometer at temperatures between phase transition and melting (422–475 K). Three experiments with different ratios of the sample mass to the volume of manometer chamber (4.7, 0.31, and 0.19 g L−1) were carried out. The results obtained for saturated vapor were independent on these ratios; therefore, the equilibrium studied was invariant. The unsaturated vapor pressure of compound 1 was measured in two independent experiments (0.31 and 0.19 g L−1). The average molecular masses of gas calculated from these experimental data (505 and 508 g mol−1) were close to the theoretical value (507.06 g mol−1); it was the evidence of the absence of other molecular forms in the gas phase. At higher temperatures (>484 K, after the melting process), the molar mass values decreased due to significant decomposition of the compound 1.
The results obtained by transmission and static methods are coincided within the method uncertainties. The enthalpy and entropy of the structural phase transition calculated using vapor pressure measurements are in good agreement with the values obtained by DSC (Table 3). This fact confirms the correctness of vapor pressure data. The significant difference between our results and those obtained by Nagaraja and co-workers [17] (Fig. 5) could be explained by the standard overstating of the vapor pressure values characterized for the thermogravimetric modification of the transmission method.
Concerning the investigation of sublimation process of complex 5 by transmission method, the two details should be noted. The first fact is the absence of difference between the mass changes in the source and receiver during all experiments. Second, the values of saturated vapor pressure are rather high at relatively low temperatures. So, the introduction of fluorinated tfac ligands instead of thd-ones (compounds 5 vs. 1) increases complex volatility and the stability of its particles in the gas phase. The quantitative volatility sequence is the following (366 K): Mg(tfac)2(tmeda) (46.3 Pa) ≫ Mg(thd)2(tmeda) (0.533 Pa). The similar correlation between volatilities of thd- and tfac-containing complexes also was observed for beta-diketonates of copper(II) [29], ruthenium(III) [30], and hafnium(IV) [31]. So, it may be supposed that in this case, the volatility of complexes is determined by the beta-diketonate ligand nature and does not depend on the nature of metal atom.
Conclusions
The present investigation was undertaken to study the influence of ligand nature on the thermal properties of magnesium complexes of Mg(beta-diketonato)2(diamine) type as promising MOCVD precursors. Herein, a series of such compounds (beta-diketonato = thd, diamine = tmeda 1, tmpda 2, pda 3; diamine = tmeda, beta-diketonato = ptac 4, tfac 5) were synthesized and characterized.
In condensed phase, Mg(thd)2(tmeda) 1 and Mg(ptac)2(tmeda) 4 have the a structural solid–solid phase transitions before melting in contradistinction to other magnesium complexes studied. For compound 1, it was shown using XRD method that this phase transition is structural. Among the complexes containing the same diamine (tmeda), compounds with fluorinated ligands 4 and 5 melt at the lower temperatures. Among the compounds containing unfluorinated beta-diketonate thd, complexes with methylated diamines melt at higher temperatures than their analogs with primary diamines instead of the diamine type.
The thermogravimetric investigation has shown that adducts with propylenediamine derivatives, i.e., Mg(thd)2(pda) 3 and Mg(thd)2(tmpda) 2, transfer into the gas phase with significant decomposition. The investigation of storage of these complexes has indicated that the process of diamine dissociation occurs. This fact is supposed to be associated with the large values of NMgN chelate angle. The magnesium adducts of fluorinated beta-diketonates (tfac, ptac) are stable toward heating and keeping.
The saturated vapor pressure investigation has shown that complex 5 with fluorinated tfac ligand is much more volatile than its thd-containing analog 1. In the transmission method conditions, complex Mg(thd)2(tmeda) 1 decomposes insignificantly up to the structural phase transition. So, the temperature range 373–413 K may be used for the evaporators of MOCVD apparatus. Then, the process of diamine dissociation proceeds more intensively; nevertheless, the vapor phase composition could be controlled as it was indicated by the investigation of unsaturated vapor pressure. The decomposition process becomes dramatic after the melting. The accuracy of the quantitative data presented for volatility of Mg(thd)2(tmeda) was confirmed by the good correlation between the results obtained by saturated vapor pressure measurements and DSC.
The information presented in this paper could be useful for obtaining of MgO thin films by MOCVD.
References
Vallet-Regi M, Labeau M, Garcia E, Cabanas MV, González-Calbet JM, Delabouglise G. Thin films of magnesium oxide by modified CVD: a buffer layer for HTCS films. Physica C. 1991;180:57–60.
Boo JH, Lee SB, Yu KS, Koh W, Kim Y. Growth of magnesium oxide thin films using single molecular precursors by metal–organic chemical vapor deposition. Thin Solid Films. 1999;341:63–7.
Wang L, Yang Y, Ni J, Stern CL, Marks TJ. Synthesis and characterization of low-melting, highly volatile magnesium MOCVD precursors and their implementation in MgO thin film growth. Chem Mater. 2005;17:5697–704.
Raj AME, Jayachandran M, Sanjeeviraja C. Fabrication techniques and material properties of dielectric MgO thin films—a status review. CIRP J Manuf Sci Technol. 2010;2:92–113.
Huang R, Kitai AH. Temperature dependence of the growth orientation of atomic layer growth MgO. Appl Phys Lett. 1992;61:1450–2.
Sung MM, Kim C, Kim CG, Kim Y. Epitaxial growth of MgO films on Si (111) by metal organic chemical vapor deposition. J Cryst Growth. 2000;210:651–4.
Davies HO, Jones AC, Leedham TJ, Crosbie MJ, Wright PJ, Boag NM, Thompson JR. Synthesis and structural characterization of a novel magnesium β-diketonatoalkoxide complex: a new precursor for the MOCVD of MgO. Chem Vap Depos. 2000;2000(6):71–5.
Putkonen M, Johansson LS, Rauhala E, Niinistö L. Surface-controlled growth of magnesium oxide thin films by atomic layer epitaxy. J Mater Chem. 1999;9:2449–52.
Matthews JS, Just O, Obi-Johnson B, Rees WS. CVD of MgO from a Mg(β-ketoiminate)2: preparation, characterization, and utilization of an intramolecularly stabilized, highly volatile, thermally robust precursor. Chem Vap Depos. 2000;6:129–32.
Hill MR, Jones AW, Russell JJ, Roberts NK, Lamb RN. Dialkylcarbamato magnesium cluster complexes: precursors to the single-source chemical vapour deposition of high quality MgO thin films. J Mater Chem. 2004;14:3198–202.
Putkonen M, Sajavaara T, Niinistö L. Enhanced growth rate in atomic layer epitaxy deposition of magnesium oxide thin films. J Mater Chem. 2000;10:1857–61.
Carta G, El Habra N, Crociani L, Rossetto G, Zanella P, Zanella A, Paolicci G, Barreca D, Tondello E. CVD of MgO thin films from bis(methylcyclopentadienyl)magnesium. Chem Vap Depos. 2007;13:185–9.
Babcock JR, Benson DD, Wang A, Edleman NL, Belot JA, Metz MV, Marks TJ. Polydentate amines as CVD precursor ancillary ligands. Epitaxial MgO thin-film growth using a highly volatile, thermally and air-stable magnesium precursor. Chem Vap Depos. 2000;6:180–3.
Carta G, El Habra N, Rossetto G, Zanella P, Casarin M, Barreca D, Maragno G, Tondello E. MgO and CaO stabilized ZrO2 thin films obtained by metal organic chemical vapor deposition. Surf Coat Technol. 2007;201:9289–93.
Hatanpää T, Kansikas J, Mutikainen I, Leskelä M. Ancillary ligand effect on the properties of “Mg(thd)2” and crystal structures of [Mg(thd)2(ethylenediamine)]2,[Mg(thd)2(tmeda)], and [Mg(thd)2(trien)]. Inorg Chem. 2001;40:788–94.
Catalano MR, Malandrino G. Multifunctional manganese single source precursor for the selective deposition of MnF2 or Mn3O4. Phys Procedia. 2013;46:118–26.
Maria M, Selvakumar J, Raghunathan VS, Mathews T, Nagaraja KS. Temperature dependence vapour pressure measurements of Mg(tmhd)2(tmeda) [(tmhd = 2,2,6,6-tetramethyl-3,5-heptanedione, tmeda = N,N,N′,N′-tetramethylethylenediamine]. Thermochim Acta. 2008;474:87–90.
Morozova NB, Zharkova GI, Stabnikov PA, et al. Synthesis and physicochemical study of b-diketonates of alkaline earth metals [in Russian]. Novosibirsk; 1989 (Preprint, Institute of Inorganic Chemistry, Siberian Branch, USSR Academy of Sciences, N 89-08).
Fadeeva VP, Tikhova VD, Nikulicheva ON. Elemental analysis of organic compounds with the use of automated CHNS analyzers. J Anal Chem. 2008;63:1094–106.
Fadeeva VP, Tikhova VD, Nikulicheva ON, Oleynik II, Oleynik IV. Composition determination of post-metallocene olefin polymerization catalysts. J Struct Chem. 2010;51:S186–91.
Nakamoto K. Infrared and Raman spectra of inorganic and organic compounds. New York: Wiley; 1997.
Basova TV, Kiselev VG, Filatov ES, Sheludyakova LA, Igumenov IK. Experimental and theoretical study of vibrational spectra of palladium(II) β-diketonates. Vib Spectrosc. 2012;61:219–25.
Kubaschewski O, Evans EL. Metallurgical thermochemistry. London: Pergamon Press; 1951.
Morozova NB, Zharkova GI, Semyannikov PP, Sysoev SV, Igumenov IK, Fedotova NE, Gelfond NV. Vapor pressure of precursors for CVD on the base of platinum group metals. J Phys IV. 2001;11:Pr3-609-616.
Zelenina LN, Titov VA, Chusova TP, Stenin YG, Titov AA. On the thermodynamic properties of germanium-iodide compounds. J Chem Thermodyn. 2003;35:1601–12.
Titov VA, Kokovin GA. In: Kokovin GA, editor. Mathematics in general thermodynamics. Novosibirsk: Nauka; 1980. p. 98–105.
Vikulova ES, Piryazev DA, Zherikova KV, Alferova NI, Morozova NB, Igumenov IK. Crystal structure of two complexes containing tris-(β-diketonato)magnate anion. J Struct Chem. 2013;54:883–9.
Sartori A, El Habra N, Bolzan M, Rossetto G, Sitran S, Barreca D, Gasparotto A, Casarin M. Stability study of a magnesium β-diketonate as precursor for chemical vapor deposition of MgO. Chem Mater. 2011;23:1113–9.
Igumenov IK, Basova TV, Belosludov VR. Volatile precursors for films deposition: vapor pressure, structure and thermodynamics. In: Mizutani T, editor. Application of thermodynamics to biological and material science. Rijeka: InTech; 2011. p. 521–46.
Morozova NB, Zherikova KV, Semyannikov PP, Trubin SV, Igumenov IK. Study of temperature dependencies of saturated vapor pressure of ruthenium(III) beta-diketonate derivatives. J Therm Anal Calorim. 2009;98:395–9.
Zherikova KV, Morozova NB, Zelenina LN, Sysoev SV, Chusova TP, Igumenov IK. Thermal properties of hafnium(IV) and zirconium(IV) β-diketonates. J Therm Anal Calorim. 2008;92:729–34.
Acknowledgements
The work was supported by RFBR grant № 12-03-31277 mol_a.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Vikulova, E.S., Zherikova, K.V., Korolkov, I.V. et al. Thermal properties of mixed-ligand magnesium complexes with beta-diketonates and diamimes as potential MOCVD precursors. J Therm Anal Calorim 118, 849–856 (2014). https://doi.org/10.1007/s10973-014-3997-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-014-3997-7