Abstract
Thermal analysis of seven Jurassic coal samples from North Shaanxi in West China and three permo-carboniferous coal samples from East China was studied to identify ignition temperatures in the process of the oxidation and spontaneous combustion. The experiments were carried out under non-isothermal heating conditions up to 700 °C at the heating rates of 5, 10, 15, and 20 °C min−1 in an air atmosphere. Through the FTIR spectrometer experiments, the absorbance peaks of functional groups of coal samples were analyzed at the ignition temperatures, pre-ignition of the 10 °C, post-ignition of the 10 °C at the heating rate of 10 °C min−1. By the differential spectrum method, the changes of functional groups were discussed with the aim to determine characteristics and reactivity of the ignition temperature around. The results showed that ignition temperatures of experimental coal samples increased with the rising heating rates, and ignition temperatures of Jurassic coals were lower than that of the permo-carboniferous coal samples at the same heating rate. Apparent activation energy of experimental Jurassic coals at the ignition temperatures was calculated by Ozawa method based on the non-isothermal and differential heating rates, ranging from 80 to 105 kJ mol−1, which were lower than that of the eastern permo-carboniferous samples. On the basis of Pearson correlation coefficient method which can signify the degree of correlations ranging from −1 to 1, the correlation analyses were conducted between activation energy and functional groups variation within 10 °C before and after ignition temperature. It was concluded that the key functional groups of Jurassic coals in the oxidation and ignition reaction were methyl and alkyl ether within 10 °C before ignition temperature, and carboxyl and carbonyl within 10 °C after ignition temperature.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coal is one of the most important materials in the energy and chemical industry, the spontaneous combustion property of which causes the wasting of coal resources and pollution of the environment in the process of oxidation and ignition. The Jurassic coal that lays in north Shaanxi coal fields of west China which is the major energy base over half of coal resources in China. Compared with permo-carboniferous coal from East China, the physics and chemistry properties and spontaneous combustion characteristic of Jurassic coal are special because of gather and degeneration rules [1, 2], which needs to be studied deeply.
In recent years, the ignition process of coal was being researched all around the world. More and more characteristics of ignition temperature were carried out. At present, the main determining methods of ignition temperature include pulse ignition technique [3, 4], laser ignition technology [5], thermal analysis technology [6–9], etc. In these methods, thermal analysis experiments could be conducted to determine the reaction kinetics parameters of coal oxidation and spontaneous combustion, which is applied widely. HU, Kök [10–12] analyzed the academic achievements of thermal analysis kinetics theory, and the different applicability of diverse thermal analysis kinetics methods. Among the kinetics methods, Ozawa [13, 14] method (also known as the iso-conversional method) was used widely to identify the reliable value of activation energy, which was based on the same conversion rate on several TG curves at different heating rates, involving the kinetic model function.
Recent researches showed that the coal was composed of several similar basic three-dimensional molecular structures, which were connected by the bridge bonds. And the reactivity of coal was closely related to these structures in the oxidation and spontaneous combustion process. Based on testing spectrum absorption intensity, FTIR spectroscopy could accurately confirm the variation and variety of functional groups during the oxidation and spontaneous combustion of coal. Cui, Ge [15, 16] studied the variation of functional groups and activity of different rank coal in the oxidation process by the method of FTIR. Kizgut [17] conducted IR spectroscopy (FTIR), thermogravimetry (TG) ,and elemental analysis experiments to characterize seven bituminous coal chars and provided suitable information to establish differences between coal samples according to their chemical compositions. Combining thermal analysis with IR spectroscopy, Kaljuvee [18] carried out TG–DTA-FTIR/MS experiments to determine thermal and kinetics characteristics of Bulgaria, Russia, Ukraine ,and other seven coal samples. He [19] characterized the ignition temperature in the process of coal oxidation and spontaneous combustion by TG–DTA-FTIR. Feng [20] studied coal structure by thermal analysis and FTIR experiments.
In this paper, Jurassic coals from North Shaanxi Coalfield of West China were studied, compared with the eastern permo-carboniferous coal samples. Thermal analysis experiments were carried out to determine the ignition temperature in oxidation and spontaneous combustion process. Based on Ozawa method at multi-heating rates, the activation energy at the ignition temperature was calculated. The variation of some active functional groups before and after ignition temperature in the coal molecular structure was analyzed by infrared spectroscopy (FTIR). And the key functional groups of Jurassic coal in North Shaanxi coalfield before and after ignition temperature were determined, which made the most contribution on spontaneous combustion.
Experimental
Coal samples
In this paper, Jurassic coal of Ning Tiaota (NTT), Yu Yang (YY), Zhang Jiamao (ZJM), Jian Zhuang (JZ), An Shan (AS), Jian Xin (JX), and Huang Ling (HL) samples from north Shaanxi coalfield, and the permo-carboniferous coal of Longgu (LG), Dongshan (DS), Bai Yangling (BYL) samples from East China were selected for the study. The experimental coal particle was grinded from 0.075 to 0.109 mm, and the proximate and ultimate analysis of coal samples were carried out, which is shown in Table 1. In the ultimate analysis experiment, the content of oxygen was determined by the subtraction method, compared with the contents of C, H, N, and S element. The experimental data showed that the characteristics of Jurassic coal were low ash, high volatile, high oxygen content, etc.
Thermal analysis
The thermal analysis experiments were performed with the German NETZSCH STA409PC DSC-TGA simultaneous thermal analyzer up to 700 °C at air atmosphere of 100 mL min−1, at heating rates of 5, 10, 15, 20 °C min−1, with the experimental sample volume of 5 mg. The TG and DSC curves with the temperature were obtained, determining ignition temperature at different heating rates and activation energy of ten coal samples.
FTIR spectroscopy experiments
The coal samples were placed inside the heating device in an air atmosphere at a heating rate of 10 °C min−1, heated to the ignition temperature, before and after ignition temperature of 10 °C, and then rapidly cooled in a vacuum oven hermetically. Using KBr compression method, coal sample and dried KBr powder were weighed in the proportion of 1:180, mixed to be grinded and put in the mold with the pressure of 20 MPa for 10 min to press into tablets. The German Brook VERTEX70 Fourier transform infrared spectroscopy was used to test the samples, with the wave number ranging from 4,000 to 400 cm−1, the resolution of 4.0 cm−1 and the accumulated scans number of 32.
Results and discussion
Determination of ignition temperature
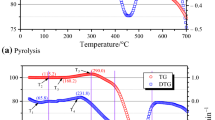
The ignition temperature of coal was the minimum point of beginning and continuing to burn when heated in an air atmosphere, which could be used as the capacity index of coal spontaneous combustion. During the thermal analysis experiments, the original adsorption equilibrium between coal samples and the gases was broken by the temperature programing, with the increasing of physical desorption of some gas. And the external water started to evaporate, which led to mass reduction. With the increasing of the heat absorbed, internal energy of coal increased. When reaching the activation of part of active structure, chemical adsorption of oxygen and chemical reaction occurred, resulting in mass increment. As the temperature continued to rise, physical desorption quantity decreased, but oxygen chemisorptions enhanced, and the mass of the experimental coal samples increased, with the oxidation and pyrolysis reactions producing CO, CO2, CH4, C2H4, etc. The mass of experimental coal samples reached to the maximum at the ignition temperature, and then the samples quickly entered into the pyrolysis and combustion stage, finally burned and maintained the same mass. Taking Yu Yang sample as an example, Fig. 1 showed the TG, DTG, and DSC curves at the heating rate of 10 °C min−1, and ignition temperature value of the experimental coal samples at different heating rates were shown in Fig. 2.
It was found that the ignition temperature of experimental coal samples increased with the rising of heating rate. High heating rate shortened the time of reaching to the same temperature, decreased the oxidation extent and caused heat transfer lag and mass transfer lag of coal, resulting in the lag of the ignition temperature. Ignition temperatures of Jurassic coal were lower than other samples at the same heating rate, indicating that Jurassic coal was easier to reach ignition temperature, and easier spontaneous combustion. And more active functional groups in molecular structures were inferred to exist in Jurassic coal.
Kinetics analysis
Under the thermal analysis conditions, it was considered that coal participated in oxidation reaction at air atmosphere. Since the experiment gas products were taken by airflow, the reaction process could be regarded as irreversible reaction. Loss (or gain) mass rate of coal in the oxidation reaction process could be expressed as integral form, and the relationship between reaction rate constants and reaction temperature (absolute temperature) could be reflected by the Arrhenius equation [10].
where α was reacted percent at the time of t; t was time; G (α) was integral form of oxidation reaction mechanism functions; A was frequency factor; E was activation energy; R was universal gas constant; T was temperature (K); β was heating rate (K min−1).
Through the transformation, Ozawa equation was expressed as follows [13]:
According to the transformed Ozawa equation, since value of α at the ignition temperature (T i) of each different heating rate (β) was equal, the value of E could be determined by the linear relationship between “logβ” and “1/T”. Fitting formula and extent of fitting (R 2) were determined by linearly fitting logβ i and 1/T i at the four heating rates. Once R 2 > 0.98, the linear fitting could be considered accurately. And compared with other methods, avoiding choosing the reaction mechanism function, Ozawa method averted the error of assuming mechanism function and could obtain E values more directly and accurately. The fitting formula, the slope ,and activation energy of each experimental coal samples are shown in Table 2. Activation energy of Jurassic coal samples in northern Shaanxi Coalfield varied from 80 to 105 kJ mol−1, lower than that of permo-carboniferous coal which ranged from 110 to 120 kJ mol−1. And the activation energy had a positive correlation with the value of ignition temperature (Fig. 3).
Jurassic coal ignition reactivity
FTIR spectrum features
Compared with the original coal sample, IR spectra at the ignition temperature could be found that the intensity of hydroxy vibration at 3300–3500 cm−1, the methyl asymmetric stretching vibration at 2920 cm−1, methylene shear vibration at 1400 cm−1 weakened. It indicated that hydroxyl, methyl, and methylene had been largely consumpted during the oxidation and spontaneous combustion, possibly due to the involving in oxidation and pyrolysis reaction. Parts of oxygen functional groups including carbonyl at 1780 cm−1 and carboxyl at 1710 cm−1 increased, the reason of which was inferred for the absorbtion of oxygen in the oxidation and pyrolysis process, and the indication was addition of mass (Figs. 4, 5).
Analysis of key functional groups
By the difference spectra method, the variation intensity of functional group within 10 °C before and after ignition temperature was determined. Carbonyl at 1780 cm−1, C=C double bond at 1600 cm−1 and some oxygen-containing functional groups of Jurassic coal increased before ignition temperature and decreased after ignition temperature, which may be attributed to the ignition of coal samples. While hydroxyl at 3300–3500 cm−1 decreased before ignition temperature, and increased after ignition temperature. It was inferred that part of the aromatic ring in coal macromolecular structure may open gradually, and oxygen-containing functional groups were consumed in the ignition process to accumulate a large number of active groups for oxidation and combustion reaction.
Absorption peak intensity value of six functional groups which changed significantly around ignition temperature was calculated, including hydroxyl vibration at 3300–3500 cm−1, methyl asymmetric stretching vibration at 2920 cm−1, carbonyl vibration at 1780 cm−1, carboxyl vibration at 1710 cm−1, C=C double bond vibration at 1600 cm−1 ,and the alkyl ethers vibration at 1040 cm−1. Combined with the absorption peak intensity value of six functional groups, correlation analysis with the activation energy was conducted to determine the key functional group of the reaction around the ignition temperature. Figures 6, 7, 8, 9, 10, 11 showed the linear relationship of carbonyl, C=C double bonds, hydroxyl, methyl, carboxyl, and alkyl ether functional groups, respectively.
The product moment correlation coefficient (R) was used to characterize the extent of Pearson correlation which was a dimensionless index ranging from −1.0 to 1.0 (including −1.0 and 1.0), could reflect the degree of linear correlation between two data sets accurately. The computational formula was as follows:
The calculated Pearson correlation coefficient is shown in Table 3. The absorption peak intensity of the carbonyl and carboxyl groups in the Jurassic coal structure increased, accumulating the material base for oxidation and ignition before ignition temperature, which lowered the value of the apparent activation energy of reaction. But after ignition temperature, the intensity of the carbonyl and carboxyl groups reduced drastically to participate in oxidation and ignition reaction. Combining the higher correlation coefficients of 0.539 and 0.633, carbonyl and carboxyl groups could be identified as the key functional groups in oxidation and ignition reaction of the North Shaanxi Jurassic coal.
Absorption peak intensity of methyl of Jurassic coal that was lower at the ignition temperature, decreased in the stage of pre-ignition, while that of the permo-carboniferous coal increased. Comparing with the correlation coefficient of functional groups and activation energy, correlation of methyl was positive (0.73) before ignition, and negative (−0.701) after ignition, showing that methyl of Jurassic coal made more contribution to the oxidation reaction which was the key functional group. Meanwhile, on the basis of the correlation analysis, alkyl ether groups could also be used as a key functional group of Jurassic coal in ignition reaction, but the contribution was less than that of methyl.
The hydroxyl absorption peak intensity of the Jurassic coal decreased before ignition temperature, and increased after ignition temperature. And the hydroxyl which was little correlation could not be considered as a key functional group of oxidation and spontaneous combustion for Jurassic coal differing from the eastern permo-carboniferous coal at the ignition temperature. The correlation of absorption peak intensity changes of C=C double bond at 1600 cm−1 and the activation energy was positive before and after the ignition temperature, but less positive at the ignition temperature, which was related with the molecular structure fracture, providing a material basis for forming secondary key functional groups in the process of pyrolysis and ignition.
Conclusions
The ignition temperature of coal samples determined by thermal analysis experiment increased with the rising heating rate. At the same heating rate, spontaneous combustion ignition temperature of Jurassic coal in Northern Shaanxi coalfield was lower than that of the permo-carboniferous coal.
Based on Ozawa method, it was observed that apparent activation energy of Jurassic coal at ignition temperatures varied in the range of 80–105 kJ mol−1, below that of the permo-carboniferous coal samples, showing that the required achieving ignition energy for spontaneous combustion was lower.
On the basis of difference spectra and Pearson correlation coefficient method, the correlation analysis was conducted between activation energy and functional groups variation within 10 °C before and after ignition temperature. It was concluded that the key functional groups of Jurassic coals in the oxidation reaction were methyl and alkyl ether within 10 °C before ignition temperature, and were carboxyl and carbonyl within 10 °C after ignition temperature.
References
Hong Zhang, Hong-tang Li, Cun-wei Xiong. Jurassic coal-bearing and accumulation regularity in Northwest China. Beijing: Geological Publishing House; 1998.
Hai-zhou Chang, Fan-gui Zeng, Wen-ying Li. Micro-FTIR study on structure of macerals from Jurassic Coals in Northwestern China. Spectrosc Spectr Anal. 2008;28:1535–8.
Wall TF, Gupta RP, Gururajan VS. The ignition of coal particles. Fuel. 1991;70:1011–6.
Zhang DK, Wall TF, Harris DJ, Smith IW, Stanmore BR. Experimental studies of ignition behaviour and combustion reactivity of pulverized fuel particles. Fuel. 1992;71:1239–46.
Chen John C, Taniguchi M, Ito K. Observation of laser ignition and combustion of pulverized coals. Fuel. 1996;74:323–30.
Meriste T, Yörük CR, Trikkel A. TG–FTIR analysis of oxidation kinetics of some solid fuels under oxy-fuel conditions. J Therm Anal Calorim. 2013;114:483–9.
Fan D, Zhu Z, Na Y. Thermogravimetric analysis of gasification reactivity of coal chars with steam and CO2 at moderate temperatures. J Therm Anal Calorim. 2013;113:599–607.
Mothé Cheila G, de Miranda Iara C. Study of kinetic parameters of thermal decomposition of bagasse and sugarcane straw using Friedman and Ozawa–Flynn–Wall isoconversional methods. J Therm Anal Calorim. 2013;113:497–505.
Babiński P, Łabojko G, Kotyczka-Morańska M. Kinetics of coal and char oxycombustion studied by TG–FTIR. J Therm Anal Calorim. 2008;28:388–91.
Hu RZ, Gao SL, Zhao XQ. Thermal analysis kinetics. Beijing: Science Press; 2008.
Kök MV. Recent developments in the application of thermal analysis techniques in fossil fuels. J Therm Anal Calorim. 2008;91:763–73.
Kök MV. Temperature-controlled combustion and kinetics of different rank coal samples. J Therm Anal Calorim. 2005;79:175–80.
Koga N. Ozawa’s kinetic method for analyzing thermoanalytical curves. J Therm Anal Calorim. 2013;113:1527–41.
Ozawa T. Applicability of Friedman plot. J Therm Anal Calorim. 1986;31:547–51.
Cui X, Zhang X, Yang. M. Study on the structure and reactivity of COREX coal. J Therm Anal Calorim. 2013;113:693–701.
Ge LM, Li JW. Evolution of functional groups in low-temperature oxidized Shenfu coal. J Xi’an Univ Sci Technol. 2003;22:187–90.
Kizgut S, Baran Y, Cuhadaroglu D. Reactivity and characterisation of various rank Turkish bituminous coal chars. J Therm Anal Calorim. 2003;71:857–65.
Kaljuvee T, Keelman M, Trikkel A, Petkova V. TG-FTIR/MS analysis of thermal and kinetic characteristics of some coal samples. J Therm Anal Calorim. 2013;113:1063–71.
Qin-lin He, De-ming Wang. Comprehensive study on the rule of spontaneous combustion coal in oxidation process by TG- DTA- FTIR technology. J China Coal Soc. 2005;30:53–7.
Jie F, Wen-ying L, Ke-chang X. Research on coal structure using FT-IR. J China Univ Min Technol. 2002;31:362–6.
Acknowledgements
This paper was supported by the Key Program of National Natural Science Foundation of China (No. 51134019), the National Basic Research Program of China (973 Program No. 2011CB411902), National Natural Science Foundation (No. 51244001), Shaanxi Innovative Research Team for Key Science and Technology (No. 2012KCT-09).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Deng, J., Wang, K., Zhang, Y. et al. Study on the kinetics and reactivity at the ignition temperature of Jurassic coal in North Shaanxi. J Therm Anal Calorim 118, 417–423 (2014). https://doi.org/10.1007/s10973-014-3974-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-014-3974-1