Abstract
Molasses is generally used as a grinding aid in cement and as a water reducer and retarder in concrete. In China, the output primarily consists of sugarcane molasses. In this paper, the effects of sugarcane molasses on the physical performance and hydration chemistry of conventional Portland cement were investigated. The setting times, the normal consistency of cement pastes, the compressive strengths and fluidities of the mortars were respectively determined according to Chinese Standard GB/T 1346, GB/T17671 and GB/T 2419. The effect of molasses on the hydration kinetics of cement was investigated using a calorimeter. The hydration products and pore size distribution of the cement pastes were analysed by X-ray powder diffraction, differential scanning calorimetry and a mercury injection apparatus. The results show that a small amount of sugarcane molasses retards the setting and hardening of cement paste and increases the fluidity of cement mortar, while excess molasses accelerates the setting and hardening. Molasses improves significantly the compressive strength at 3d due to the decrease of porosity. The addition of 1.0 % molasses accelerates the formation of ettringite, prevents the second hydration of aluminate phase and delays the hydration of C3S.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Molasses is a viscous by-product of the refining of sugarcane, grapes, or sugar beets into sugar. The composition of molasses is variable and is dependent on the peculiarity of the raw materials and the manufacturing conditions. Molasses can be used as fodder for livestock and as retarding admixture, water-reducing admixture and grinding aid for construction materials. In Western countries, the majority of molasses has been used as fodder without adverse environment problems. However, in China, molasses has been extensively employed as a grinding aid and a water-reducing and retarding admixture in cement and concrete [1, 2]. Xiaojian Gao et al. utilised beet molasses as a grinding aid and proposed that the addition of 0.01–0.05 % beet molasses by cement weight could improve the particle size distribution, which results in the strength development of blended cement [3]. Amanmyrat suggested that beet molasses could significantly increase both initial and final setting times, slightly increase the strength of concrete at all ages, with the exception of concrete at an early age, and exhibit no adverse effects on durability properties over an extended period (900 days) [4]. Some researchers have provided different explanations regarding the retarding mechanism. The retarding action is generally attributed to the adsorption of molasses on the surfaces of hydrating cement particles and/or hydration products [5].
In China, the majority of cement plants use a grinding aid to increase production or to improve the early strength of cement. Consequently, the adaptability of cement to superplasticiser has deteriorated. The use of a grinding aid causes an increase in fine particles, which accelerates the hydration of cement. A specific amount of superplasticiser is adsorbed on the hydrated products to reduce its action. Thus, a retarding agent is necessary to delay the hydration of cement. The sugar in molasses is an effective retarder. Hence, a detailed investigation of the influence of sugarcane molasses on the physical properties and hydration kinetics of cement is required. The setting time, fluidity, development of mechanical strength, hydration kinetics and microstructure of cement both with and without sugarcane molasses were analysed. The required amount of sugarcane molasses was proposed. The results are helpful for improving the adaptability of cement to superplasticiser.
Experimental
Materials
Raw materials
The materials used in the experiments included conventional Portland cement without a grinding aid, standard sand and sugarcane molasses. The chemical composition of the cement, which was obtained from the Yangzhou Yadong Cement Co., Ltd., is shown in Table 1. The surface area of the cement is 360 m2 kg−1. The standard sand (Chinese Standard GB/T17671) was obtained from the China ISO Standard Sand Co., Ltd.
Paste and mortar
Pastes were prepared with cement and water. A water-to-cement ratio of 0.29 was used to investigate the effect of molasses on setting times, hydrated products and pore distribution. A water-to-cement ratio of 0.5 was used to investigate the hydration kinetics of cement. Mortars were prepared with standard sand in accordance with Chinese Standard GB/T17671 for compressive strength and fluidity. Cement/sand/water mass ratios of 1:3.0:0.5, respectively, were employed.
Test
The setting times and the normal consistency of cement pastes were determined in accordance with Chinese Standard GB/T 1346 using a Vicat apparatus. The effect of molasses on the hydration kinetics of cement was investigated using a calorimeter (TAM Air from Thermometric AB, Sweden, at 20 °C). The hydration products and pore size distribution of the cement pastes were analysed by X-ray powder diffraction (Japan Rigaku International Corporation SmartLab x; working condition: 40 kV, 30 mA, Cu Kα); differential scanning calorimetry (DSC) (NETZSCH STA-449C equipment, a heating rate of 10 °C/min from 50 to 527 °C under N2 atmosphere), and with mercury injection apparatus (Quanta chrome Pore Master GT60). The compressive strengths and fluidities of the mortars were measured according to Chinese Standards GB/T17671 and GB/T 2419.
Results and discussion
Normal consistency and setting times for cement pastes
The required amounts of water and setting times for the cement pastes are shown in Fig. 1. Sugarcane molasses influences the amount of water that is required to achieve a normal consistency for cement. The amount of required water increases with a small addition of molasses. However, the amount of required water decreases significantly, if the amount of molasses added is 1.0 % by mass of cement. Molasses increases the water requirement due to the development of cement particle fineness and specific surface when beet molasses is used as a grinding aid in the amount of 0.02 or 0.03 % by mass of cement [3]. In this study, molasses dissolved in solution was directly added to the cement. Ashworth showed that sugar can be used as a surface-active agent to improve the workability of concrete [6]. For sugar dosages of 0.03 and 0.06 %, the required amount of water decreased by 4 and 5 %, respectively [7]. As sugarcane molasses contains a high percentage of sugar, the reduction in required water is partly attributed to the water-reducing effect of sugar.
As shown in Fig. 1, the initial and final setting times were delayed by <1.0 % molasses for the cement; this result was most likely attributed to the sugar in sugarcane molasses. Numerous types of sugar, such as sucrose and glucose, have been reported to be excellent retarders [8]. The retarding effect of beet molasses exhibited a significant improvement in setting times [3]. But when the amount of molasses added reaches to 1.0 %, the setting times of cement paste are shortened. It may be correlated with the electrochemistry change caused by molasses. Singh and Yang showed that the zeta potential of hydrating cement is changed from positive to negative due to sugar [9, 10].
Fluidity of cement mortars
The fluidities of cement mortars are shown in Fig. 2. For the same water-to-cement ratio, cement mortars with sugarcane molasses show higher fluidities compared with the blank cement mortar at any hydration time. For Portland cement without an admixture, the fluidity of the cement mortar should decrease with an increase in the required water for a normal consistency of cement paste. However, a significant difference in fluidities has been demonstrated in this paper due to the addition of molasses. Although a small amount of sugarcane molasses increases the fluidity of cement mortars, it increases the required water for a normal consistency of cement paste. Young proposed that sugar bonded to the aluminate phase in cement by complexing or chelating and promoted the dissolution of ions from the hydrating calcium-silicate phase [11]. Thomas and Birchall also revealed high calcium and hydroxide ion concentrations and silicon, aluminate and iron concentrations that were 500 times higher for cement pastes with 50 mM sucrose compared with neat cement pastes [12–14]. Sugar enables ions to coexist in solution at higher concentrations without precipitation. In this paper, the increase in required water may be ascribed to rapid dissolution of the cement with molasses. Molasses does not reduce the amount of free water. Hence, it improves the fluidity of cement mortar.
For equivalent hydration times, the fluidities of cement mortars decrease slightly when the addition of sugarcane molasses achieves saturation. With an increase in hydration time, the saturated amount of sugarcane molasses is enhanced. This finding demonstrated that the reaction of sugarcane molasses with hydrated products resulted in the consumption of molasses during hydration. This topic requires further investigation.
Compressive strengths of cement mortars
The compressive strengths of mortars prepared with different amounts of sugarcane molasses are shown in Fig. 3. Mortars with sugarcane molasses yield higher strengths compared with the blank mortars at an age of 3d. The compressive strengths of mortars increase with the amount of sugarcane molasses. In particular, an approximate 12 % increase in compressive strength was obtained for the mortar that contained 0.04 % molasses compared with the blank mortar. As previously discussed, Amanmyrat suggested that beet molasses can improve the early strength of concrete due to a more uniform and dense structure of concrete with beet molasses under prolonged hardening [4]. In addition, the early strength of concrete with molasses was higher compared with concrete that contained an effective retarder due to the chloride contained in molasses [15]. At an age of 28 days, slight increases were obtained for the mortars with 0.03, 0.04 or 0.05 % molasses. However, the compressive strengths decreased for mortars with 0.01 or 0.02 % sugar molasses. Juenger and Jennings proposed that sugar can increase the surface areas of cement pastes cured at 40 °C in the hydration range of 0.55–0.80 due to a variation in the microstructure of C–S–H [5].
Hydration kinetics
The heat flow curves and cumulative heat curves for cement pastes are shown in Fig. 4. The heat flow curve reflects the hydration rate of cement. The hydration of cement involves several distinct and transient processes [16]. These processes generally fall within the following categories: (1) the dissolution of molecular species from cement particle surfaces, (2) the diffusion of these species within an aqueous solution or near solid surfaces, (3) the precipitation or nucleation of hydration products in solution or on solid surfaces, and (4) the growth of disordered or crystalline hydration products. Regarding the heat flow curves for cement pastes, the first peak is ascribed to the dissolution of ions from solid surfaces and the formation of ettringite (AFt), the second peak is attributed to the hydration of alite, and the shoulder on the second peak is attributed to the second hydration of the aluminate phase to form the monosulphoaluminate phase. Regarding the effect of sugarcane molasses on the hydration kinetics of cement paste, sugarcane molasses prolongs the duration of the induction period, in particular 1.0 % molasses (Fig. 4a). The second exothermic peak did not appear after the cement paste with 1.0 % sugarcane molasses had been hydrated for 3d. Hence, sugarcane molasses significantly inhibits the hydration of alite and the second hydration of aluminate phase. Young proposed that sugar retards hydration due to an increase in the solubility of ions from cement particles and their subsequent adsorption on portlandite (CH) and hydrated calcium silicate (C–S–H) to prevent their growth [4].
To investigate the effect of sugarcane molasses on the initial hydration of cement, the initial heat flow was measured by internal mixing (Fig. 4b). Increasing the dosages of sugarcane molasses hinders the wetting of cement particles by water. Although a small amount of molasses does not change the hydration kinetics of cement, it enhances the maximum rate of liberation heat. When 1.0 % molasses is added, the maximum rate of liberation heat decreases and endures longer compared with blank cement. It may be ascribed that excess molasses promotes the nucleation and growth of AFt. A small amount of molasses could promote the dissolution of cement. During deceleration, the hydration rate is higher for cement with 1.0 % molasses compared with the hydration rate of the other samples. The cumulative heat reflects the hydration degree (Fig. 4c). Within 3d of hydration, the hydration degrees of the cement pastes with molasses are low compared with the blank cement paste at any time, except for the cement paste with 1.0 % molasses. Prior to 2.5 h of hydration, the cumulative heat of the cement paste with 1.0 % molasses is significantly higher, compared to the remaining pastes. The experimental evidence indirectly demonstrates that a small amount of molasses could accelerate the dissolution of aluminate phase, and excess molasses could accelerate the nucleation and growth of AFt.
Phase compositions of cement pastes
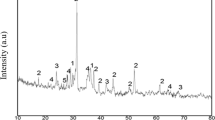
The phase compositions of cement pastes were analysed by X-ray powder diffraction. The cements with and without molasses were hydrated respectively for 30 min, 1, 3, 5, 24 h, 3 and 28d. Figure 5 just shows the XRD patterns for cement pastes hydrated at 3d. At 30 min, AFt has formed for the cement paste with 1.0 % sugarcane molasses, and CaSO4·2H2O has been consumed at 5 h. With the ongoing hydration, the main phase compositions consist of AFt, CaSO4·2H2O, unhydrated C3A, C3S, C2S and Ferrite for cement hydrated for 1 h. At 3 h, Ca(OH)2 has begun to form due to the hydration of Alite for the cement pastes, except for the cement pastes with 0.1 and 1.0 % molasses, while it did not form until 28d for cement pastes with 1.0 % sugarcane molasses. Hence, excess sugarcane molasses significantly delays the hydration of C3S at the early time. This finding is consistent with the heat flow.
To clarify the effect of molasses on the hydration of C3A and Ferrite phases, the characteristic peaks of AFt, CaSO4·2H2O, C3A and Ferrite were selected. The peaks for cement hydrated at 1, 5 h and 1d are just shown in Fig. 6. At 30 min, 1, 3 and 5 h of hydration, the peak intensity of AFt is higher for the cement paste with 1.0 % molasses, while the peaks intensities of C3A and Ferrite are lower, compared to the remaining pastes (see Fig. 6a, b). CaSO4·2H2O is almost depleted for the cement with 1.0 % molasses, which was hydrated at 5 h(see Fig. 6b). This result demonstrates that sugarcane molasses can accelerate the reaction of C3A, ferrite and CaSO4·2H2O at an early hydration time and presents evidence for the more cumulative heat of the cement paste with 1.0 % molasses prior to 5 h of hydration. These results correspond with the results of L.M. Meyer and M. Bishop [17, 18]. At hydration of 1d (see Fig. 6(c)), the peak intensities of AFt, C3A and Ferrite are higher for the cement paste with 1.0 % molasses compared with the other pastes. It can be concluded that CaSO4·2H2O is consumed in the range of 5–24 h for the cement pastes, except for the cement paste with 1.0 % molasses. Subsequently, the left C3A reacts with AFt to form monosulphoaluminate (AFm). An amount of 1.0 % molasses prevents the left C3A from reacting with AFt. Some C3A remains even though the cement with 1.0 % molasses was hydrated for 28d.
The result from the DSC further proves the affect of molasses on the hydration of conventional Portland cement (see Fig. 7). Two peaks in the range of 50–300 °C were observed in DSC curves for cements hydrated for 3d. The first endothermic peak at about 100 °C is ascribed to the dehydration of C–S–H and AFt, and the second endothermic peak at about 430 °C is attributed to the dehydration of Ca(OH)2. There is no endothermic peak at about 430 °C for the cement paste with 1.0 % molasses. It agrees with the result form XRD. Hence, the endothermic peak at about 100 °C just belongs to the dehydration of AFt for the cement paste with 1.0 % molasses. And the content of AFt is more, compared to the other pastes.
According to the previous statement, sugar absorbs on the surface of C3A. When a small amount of molasses is added, molasses is unable to prevent the hydration of C3A due to bonding to the aluminate phase by complexing or chelating. Garci Juenger and Jennings suggested that sugar generally absorbs to the surfaces of hydrating cement particles and/or the surfaces of hydration products [5]. Benjamin J. Smith proposed that sucrose is stable in alkaline cement slurries and exhibits selective adsorption on hydrating silicate surfaces, but not on aluminate surfaces in cement [19]. Ramachandran et al. noted that cement that contains high aluminates requires a greater amount of sugar to achieve an equivalent retardation, which suggests that sugar adsorbs on aluminates [20].
Pore porosity and distribution of cement pastes
The pore size distribution and cumulative pore volumes of cement hydrated at 3d are shown in Fig. 8. The pore sizes of the cement pastes with molasses are smaller compared with the pore sizes of the frank cement paste. The corresponding cumulative pore volumes also decrease. These results suggest that the addition of molasses effectively decreases the pore porosity and improves the density of cement paste. The decreased pore diameter is favourable to strength development (as shown in Sect. 3.3) and the durability improvement of cements and concretes [21]. The reduction in pore size is due to the gradual filling of large pores, which is caused by hydration reactions and the packing effect. According to these results, a small amount of molasses can improve the packing effect for initial times, but does not increase the amount of hydrated products. Thus, the higher strengths of the previous concretes can be attributed to the more uniform and dense structures formed under prolonged hardening.
Conclusions
A small amount of sugarcane molasses increases the required amount of water for the normal consistency of cement paste and the fluidity of cement mortar. A small amount of molasses can be used as a retarder in cement to retard the setting time of cement paste. However, 1.0 % molasses reduces the required amount of water and accelerates the setting and hardening of cement pastes.
The duration of the induction period is prolonged due to an increased amount of molasses. A small amount of sugarcane molasses could accelerate the dissolution of aluminate phase, and excess molasses could promote nucleation and growth of AFt at the initial time. The formation of AFt from the hydration of C3A and Ferrite is accelerated by 1.0 % molasses before 5 h. But the second hydration of C3A and Ferrite is prevented significantly, since the hydration of 1d for the cement paste with 1.0 % molasses. At the same time, the hydration of C3S is delayed and does not form until 28d for the cement paste with 1.0 % molasses.
A small amount of sugarcane molasses enhances the compressive strength of cement mortar at 3d, which is ascribed to the notion that a small amount of molasses lowers the porosity and pore size for obtaining a denser structure for cement pastes compared with blank cement paste.
References
Olbrich H. The molasses. Berlin: Biotechnologie-Kempe GmbH; 2006.
Liu J, Ran Q, Zhang J, Jiang J. Study on molasses in retarding and water reducing properties of cement pastes. N Build Mater. 2012;02:38–41.
Xiaojian G, Yang Y, Deng H . Utilization of beet molasses as a grinding aid in blended cements. Constr Build Mater. 2011;25(9):3782–9.
Jumadurdiye A, Hulusi Ozkul M, Saglam AR, Parlak N. The utilization of beet molasses as a retarding and water-reducing admixture for concrete. Cem Concr Res. 2005;35(5):874–82.
Maria C, Juenger G, Jennings HM. New insights into the effects of sugar on the hydration and microstructure of cement pastes. Cem Concr Res. 2002;32(3):393–9.
Ashworth R. Some investigations into the use of sugar as an admixture to concrete. Proc Inst Civ Eng. 1963;31:129–45.
Tuthill LH, Adams RF, Hemme JM. Observations in testing and use of water-reducing retarders. ASTM Spec Publ. 1960;266:97–117.
Bolobova AB, Kondrashchenko VI. Use of yeast fermentation waste as a biomodifier of concrete (review). Appl Biochem Microbiol. 2000;36(3):243–53.
Singh NB, Ohja PN. Effect of glucose on the hydration of Portland cement. In: Proceedings of the 7th International Congress on the Chemistry of Cement II Editions. Paris: Septima; 1980. p. 100–105.
Yang M, Neubauer CM, Jennings HM. Interparticle potential and sedimentation behavior of cement suspensions. Adv Cem Based Mater. 1997;5(1):1–7.
Young JF. A review of the mechanisms of set-retardation in portland cement pastes containing organic admixtures. Cem Concr Res. 1972;2(4):415–33.
Thomas NL, Birchall JD. The retardation action of sugars on cement hydration. Cem Concr Res. 1983;13(6):830–42.
Thomas NL, Birchall JD. A Reply to a Discussion by S. Chatterji of ‘The retarding action of sugars on cement hydration’ by N.L. Thomas and J.D. Birchall. Cem Concr Res. 1984;14(5):761–2.
Birchall JD, Thomas NL. The mechanism of retardation of setting of OPC by sugars. Proc Br Ceram Soc. 1984;35:305–15.
Ramachandran VS, Lowery MS. Conduction calorimetric investigation of the effect of retarders on the hydration of Portland cement. Thermochim Acta. 1992;195(17):373–87.
Bullard JW, Jennings HM, Livingston RA, Nonat A, Scherer GW, Schweitzer JS, Scrivener KL, Thomas JJ. Mechanisms of cement hydration. Cem Concr Res. 2011;41(12):1208–23.
Meyer LM, Perenchio WF. Theory of concrete slump loss as related to use of chemical admixtures. ACI Concr. Int. 1979;1(1):36–43.
Bishop M, Barron AR. Cement hydration inhibition with sucrose, tartaric acid and lignosulfonate: analytical and spectroscopic study. Ind Eng Chem Res. 2006;45(21):7042–9.
Smith BJ, Roberts LR, Funkhouser GP, Gupta V, Chmelka BF. Reactions and surface interactions of saccharides in cement slurries. Langmuir. 2012;28(40):14202–17.
Ramachandran VS, Feldman RF, Beaudoin JJ. Concrete science, treatise on current research. London: Heyden; 1981.
Glasser FP, Marchand J, Samson E. Durability of concrete—degradation phenomena involving detrimental chemical reactions. Cem Concr Res. 2008;38(2):226–46.
Acknowledgements
The authors are grateful for the financial support of the National Natural Science Foundation of China (51202109), and the Program for Changjiang Scholars and Innovative Research Team in University (IRT1146). The support by the facilities of the Modern Analysis and Testing Centre at NJTECH, where the detailed microstructural analyses were performed, is also acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Weifeng, L., Suhua, M., Shengbiao, Z. et al. Physical and chemical studies on cement containing sugarcane molasses. J Therm Anal Calorim 118, 83–91 (2014). https://doi.org/10.1007/s10973-014-3947-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-014-3947-4