Abstract
The effect of several kinds of antioxidants on the stability of poly(vinyl butyral) (PVB) under air atmosphere is studied by thermogravimetry–differential scanning calorimetry method and kinetic analysis. After mixed with antioxidants, the thermal oxidative stability of PVB increases significantly, because the antioxidants could inhibit the oxidation of copolymer (stage I). The thermal oxidative stability increases in the following order: PVB < PVB/1010 < PVB/B215 < PVB/1098. However, the thermal oxidative degradation rate of PVB increases markedly after 320 °C, due to the loss of chemical activity for antioxidants gradually. The thermal stability of antioxidants increases in the following order: B215 < 1010 < 1098.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Poly(vinyl butyral) (PVB) is extensively used in glass lamination and highly susceptible to oxidation. Exposure to air, heat, light, or metal contaminants accelerates oxidative reactions. Antioxidants could increase the oxidation induction period or decrease the rate of oxidation. Antioxidants 1010, 1098, and B215 are used in the polymer industries. The structures of these compounds allow for the donation of protons to free radicals, thus inhibiting the oxidative process.
Recently, different combinations of analytical tools have been used to analyze the stability and thermal properties of polymers. In 2012, Santos [1] used a thermogravimetry–differential scanning calorimetry (TG–DSC) method to determine the thermal stability and oxidation stability of several antioxidants like commercial synthetic antioxidants: TBHQ, BHT, BHA, and PG, as well as natural antioxidants: α-tocopherol, caffeic acid, and ferulic acid. The results showed that thermal resistance of these synthetic compounds decreased in the following order: PG > TBHQ > BHA > BHT, while thermal resistance of the natural antioxidants decreased in the following order: α-tocopherol > caffeic acid > ferulic acid > gallic acid.
In 2010, Stephanie Etienne [2] used the thermogravimetry–differential scanning calorimetry–Fourier transform infrared spectroscopy (TG–MS–FTIR) system to study the thermal properties of poly(vinyl chloride) (PVC) samples which incorporated with PVB matrix and calcium carbonate nanoparticles. It showed that both PVB and CaCO3 nanoparticles acted as HCl scavengers and afforded a significant delay of both onset degradation temperature and HCl release.
Lorıa-Bastarrachea [3] used a TG–FTIR and HNMR system to study the thermal degradation of polyvinyl pyrrolidone (PVP), the results showed that vinyl pyrrolidone was the main volatile production from the thermal degradation of PVP which could imply that the predominant process during thermal degradation of this polymer is the broken of polymer chains.
In 2006, Fernandez [4] used a TG–DSC–FTIR and NMR method to study the thermal stability in nitrogen of the a new polymer PVB by the acetalization of PVA with butyraldehyde. The result showed that the thermal stability depended on the content of the hydroxyl groups, increasing with decreasing this content. PVB with less than 80 % in mol VA units are more thermally stable and PVB with 82 % in mol is less stable.
In 2004, Liau [5] used a TG-FTIR system to study the thermal degradation of PVB/glass ceramic/Ag composites in nitrogen and air environments, the result showed that both of these inorganic materials have only a slight effect on PVB thermal degradation in nitrogen, but under air atmosphere a strong catalytic effect of both inorganic materials on PVB thermal degradation was observed.
In 2002, Dhaliwal [6] used TG and 13C NMR to characterize the PVB obtained from a wide range of sources. The result showed that most recycled PVB samples had similar glass transition temperature, but these could be adjusted by the addition of further amounts of dibutyl sebacate.
Antioxidants 1098, 1010, and B215 are the synthetic antioxidants that are used in PVB industry. However, the effect of these antioxidants in air atmosphere in PVB was not studied in the past. This paper deals with the effect of the antioxidants on the stability of PVB which is measured by thermal analytical techniques.
Experimental
Materials
Irganox 1010 and 1098 are sterically hindered phenolic antioxidants, and Irganox B215 is a kind of complex antioxidant by mixing Irganox 1010 with Irganox 168. Commercial antioxidant types B215, 1010, and 1098 from BASF Global were used in this work. And commercial PVB sample type Butvar resin (B-72) was obtained from Solutia Inc. The average molecular mass of PVB is between 170,000 and 250,000, approximately.
The samples were composed of pure PVB and antioxidant. The composition of the samples is listed in Table 1.
Apparatus and method
A Netzsch STA 449C TG–DSC Unit, interfaced to a PC and a temperature programmer, was used to determine the mass loss and thermal change of the PVB samples during the experiment. Approximately 4 mg of sample was heated over a temperature range from ambient to 700 °C in flowing air atmosphere under 20 mL min−1. It was used dynamically at 10, 15, 20, 25, and 30 K min−1, and the samples were carried in a Al2O3 crucible.
Results and discussion
The thermal oxidative stability of PVB
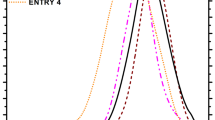
Figure 1 shows the results of the TG–DSC curves at 10 K min−1 heating rate for the pure PVB, PVB/B215, PVB/1098, and PVB/1010 under air atmosphere.
The DSC curve of pure PVB shows four-step thermal oxidative degradation in air atmosphere. Liau [7] thought that the thermal oxidative degradation of individual PVB consisted of oxidation of copolymer (7 % mass loss), PVB thermal oxidation (71 % mass loss), oxidation of cyclic and crosslinked compounds (9 % mass loss), and oxidation of residual carbon (13 % mass loss). The process of thermal oxidative degradation of PVB/antioxidants mixtures could also be divided into the same four stages.
Table 2 lists the temperature range of the different stages of PVB thermal oxidative degradation. The temperature range of stage I of pure PVB thermal oxidative degradation is 207–298 °C, much lower than that of mixtures, about 260–320 °C. The end temperature of stage II of mixtures (about 460 °C) is slightly higher than that of the individual PVB (455 °C). And the end temperature of stage III of mixtures (about 550 °C) is higher than that of the pure PVB (536 °C). It is revealed that the antioxidants inhibit the oxidation of copolymer significantly between 260 °C and 320 °C. Then, the antioxidants decompose or volatilize after 320 °C, and the antioxidants lose the chemical activity gradually.
Table 3 lists the temperatures at 2 % mass loss (T TG2 %), onset reactive temperatures (T DSC), the temperature of the exothermic peak (T p) of the main reaction, and the temperature of the maximum thermal oxidative degradation rate (T max).
The T TG2 % of mixtures is about 300 °C and the T DSC of mixtures is about 270 °C, which is higher than those of individual PVB. And it increases in the following order: PVB < PVB/1010 < PVB/B215 < PVB/1098. The results indicate that the thermal oxidative stability of the samples increases in the following order: PVB < PVB/1010 < PVB/B215 < PVB/1098.
The T p and T max of mixtures are about 390 °C and 380 °C, respectively, which is higher than that of individual PVB. And it increases in the following order: PVB < PVB/B215 < PVB/1010 < PVB/1098. As the decomposition or volatilization of antioxidants, the antioxidants lose chemical activity leading to the increase of the rate of thermal oxidative degradation for PVB. The results indicate that the thermal stability of antioxidants increases in the following order: B215 < 1010 < 1098.
Kinetic of thermal oxidative degradation
There are two ways to carry out the kinetic analysis of the thermal oxidative degradation of PVB based on nonisothermal operations. The general rate equation for polymer thermal degradation can be written as
α is the conversion, T is the temperature, A is the frequency factor, β is the heating rate, R is the gas constant, E is the activation energy, and f(α) is the conversional functional relationship.
For polymer thermal oxidative degradation, it is assumed that the rate constant is proportional to the concentration of nondegradation material [4]
n is the apparent reaction order.
Kissinger method
The kinetic parameters can be obtained from Kissinger method [8] using the temperature (T max) at which the rate of mass loss is maximum, and these parameters are assumed to be independent of β. After a series of conversions, following expression is obtained
Figure 2 shows good linear relationship plots between \( { \ln }\left( {\frac{\beta }{{T_{ \hbox{max} }^{2} }}} \right) \) and \( \frac{1}{{T_{ \hbox{max} } }} \) for PVB, PVB/1010, PVB/1098, and PVB/B215. And the apparent activation energy and frequency factor for each sample could be obtained by these Kissinger plots.
Table 4 lists the activation energy, frequency factor, and the linear regression coefficient of all samples. The activation energy of pure PVB is 131.85 kJ mol−1, which is much lower than that of mixtures. It is indicated that antioxidants inhibit the oxidation of PVB significantly. And the activation energy of samples increases in the following order: PVB < PVB/1010 < PVB/B215 < PVB/1098. The results indicate that antioxidative ability of Irganox increases in the following order: 1010 < B215 < 1098.
Ozawa–Flynn–Wall method
The model-free Ozawa–Flynn–Wall method [9] was used to analyzed the reaction process.
where \( G\left( \alpha \right) = \frac{A}{\beta }\int_{0}^{T} {{\text{e}}^{\text{ - E/RT} }} {\text{d}}T = \frac{AE}{\beta R}\int_{\infty }^{u} {\frac{{ - {\text{e}}^{\text{ - u} }}}{{u^{2} }}} {\text{d}}u = \frac{AE}{\beta R}P\left( u \right). \)
Using Doyle’s approximation we have \( { \lg }P_{D} \left( u \right) = - 2.315 - 0.4567\frac{E}{RT} \), β is the heating rate. A is the frequency factor, R is the gas constant, α is the conversion, T is the absolute temperature and u is \( \frac{E}{RT} \) .
The Netzsch Thermokinetic software and the nonisothermal data at the heating rate of 10, 15, 20, 25, and 30 K min−1 were used to calculate the activation energy of all samples, then the apparent activation energy of each mixture was compared with the result of pure PVB. The result is shown in Fig. 3.
The results show that the activation energy of thermal oxidative degradation of PVB/1010 and PVB/1098 is higher than that of individual PVB at the mass loss of 1–20 %. The activation energy of thermal oxidative degradation of PVB/B215 is lower than that of individual PVB at the mass loss of 1–9 %, and higher than ones of individual PVB at the mass loss of 10–20 %. It is revealed that the antioxidants could increase the thermal oxidative stability of PVB at the mass loss 10–20 %.
Conclusions
Based on the investigation of the impacts of different antioxidants on the thermal oxidative degradation of PVB, the following conclusions were drawn.
-
(1)
After mixed with antioxidants (1010, 1098, and B215), the thermal oxidative stability of PVB increases significantly. And the values of T TG2 %, T DSC, T p, T max and activation energy go up, respectively.
-
(2)
The antioxidants could inhibit the oxidation of copolymer (stage I). And after 320 °C, the antioxidants lose their chemical activity gradually. The thermal oxidative degradation rate of PVB is increased markedly.
-
(3)
The ability of different antioxidants of inhibiting thermal oxidative degradation of PVB increases in the following order: 1010 < B215 < 1098. And the thermal stability of antioxidants increases in the following order: B215 < 1010 < 1098.
References
Santos NA, Cordeiro AMTM, Damasceno SS, et al. Commercial antioxidants and thermal stability evaluations. Fuel. 2012;97:638–43.
Etienne S, Becker C, Ruch D, et al. Synergetic effect of poly(vinyl butyral) and calcium carbonate on thermal stability of poly(vinyl chloride) nanocomposites investigated by TG–FTIR–MS. J Therm Anal Calorim. 2010;100(2):667–77.
Lorıa-Bastarrachea MI, Herrera-Kao W, Cauich-Rodrıguez JV, et al. A TG/FTIR study on the thermal degradation of poly(vinyl pyrrolidone). 2010.
Fernandez MD, Fernandez MJ, Hoces P. Synthesis of poly(vinyl butyral)s in homogeneous phase and their thermal properties. J Appl Polym Sci. 2006;102(5):5007–17.
Liau LCK, Liau JY. Kinetic effects of glass ceramic and silver on poly(vinyl butyral) thermal degradation using FT-IR. 1. Ind Eng Chem Res. 2004;43(9):1938–43.
Dhaliwal AK, Hay JN. The characterization of polyvinyl butyral by thermal analysis. Thermochim Acta. 2002;391(1):245–55.
Liau LCK, Yang TCK, Viswanath DS. Mechanism of degradation of poly(vinyl butyral) using thermogravimetry/Fourier transform infrared spectrometry. Polym Eng Sci. 1996;36(21):2589–600.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29(11):1702–6.
Flynn JH, Wall LA. General treatment of the thermogravimetry of polymers. J Res Nat Bur Stand. 1966;70(6):487–523.
Acknowledgements
This work was supported by the Fundamental Research Funds for the National Natural Science Foundation of China (NSFC51202113) and Central Universities (NUST 2011 YBXM10).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mei, X., Jiao, S., Li, X. et al. Effect of the antioxidants on the stability of poly(vinyl butyral) and kinetic analysis. J Therm Anal Calorim 116, 1345–1349 (2014). https://doi.org/10.1007/s10973-014-3806-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-014-3806-3