Abstract
It has been shown, for three different polymer layered silicate (PLS) nanocomposite systems, how differential scanning calorimetry (DSC) can identify the different reactions of homopolymerisation and of crosslinking that occur in the intra- and extra-gallery regions of these nanocomposites, respectively, and hence how DSC can be used to assess the cure conditions for optimising their nanostructure. The PLS nanocomposites are based upon: (i) diglycidyl ether of bisphenol-A (DGEBA) cured with a polyoxypropylene diamine; (ii) DGEBA cured with an –NH2 terminated hyperbranched polymer (HBP); and (iii) tri-glycidyl p-amino phenol (TGAP) cured with a diamine. In each case, the existence of both intra- and extra-gallery reactions in the DSC cure curves, and whether they occur simultaneously or sequentially, and in what order, are identified and correlated with the nanostructure as observed by small angle X-ray scattering and transmission electron microscopy. In particular, it is shown that the intra-gallery reaction must precede the extra-gallery for significant exfoliation to occur. In accordance with this scenario, the TGAP/diamine system displays the greatest degree of exfoliation, the DGEBA/diamine system the least, with the DGEBA/HBP system intermediate. For those systems in which significant exfoliation occurs, the DSC cure curves also allow the optimum cure conditions, such as the isothermal cure temperature, to be determined.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polymer layered silicate (PLS) nanocomposites based upon epoxy resin are generally fabricated by intercalating the epoxy resin into the clay galleries, and then adding the crosslinking agent and curing the nanocomposite [1–3]. Ideally, this gives an exfoliated nanostructure, which may be identified by small angle X-ray scattering (SAXS) and transmission electron microscopy (TEM), but in practise this is difficult to achieve, and in many cases authors refer instead to “partially exfoliated” nanocomposites when the SAXS data suggest that there is no layer stacking with a d-spacing less than about 8 nm [e.g., 4–6]. Nevertheless, even when TEM shows regions in which the clay layers are widely separated and not in register, characteristic of a partially exfoliated nanocomposite, there always remain some other regions in which layer stacking is evident [4–11].
It is generally agreed that in order to obtain a high degree of exfoliation, the intra-gallery reaction must occur before the extra-gallery crosslinking reaction [5, 12–16]; if the extra-gallery reaction occurs first, then the network formed in the bulk of the nanocomposite prevents further nanostructural development in the clay galleries. Indeed, in the latter case it is even possible for the resulting d-spacing of the clay layers to be less than that of the epoxy-intercalated clay before curing, as a consequence of the shrinkage of the bulk resin during cure [16, 17]. Although these effects are clearly shown by the nanostructural characterisation techniques such as SAXS and TEM, we show here that differential scanning calorimetry (DSC) also can identify these different reactions taking place during cure. Hence DSC is not only a complementary technique to SAXS and TEM but it can also be used both to assess the cure conditions for optimising the nanostructure of PLS nanocomposites, and to obviate the need for nanostructural characterisation by SAXS and TEM in situations in which DSC shows that the intra-gallery reaction occurs after the extra-gallery reaction, and hence that there will be poor exfoliation. A relatively recent review [18] presents examples of situations in which thermal analysis in general, including DSC, temperature modulated DSC, dynamic mechanical thermal analysis, and thermogravimetric analysis, have been used in the characterisation of PLS nanocomposites, for the most part based upon thermoplastic polymers as the matrix material. In the present paper, we illustrate this by particular reference to the use of DSC and to the preparation of three different PLS nanocomposites based upon epoxy resin.
Experimental
Materials
Two epoxy resins were used in this study: diglycidyl ether of bisphenol-A (DGEBA), DER 331 (Dow Chemical Company), a commercial epoxy resin with an epoxy equivalent in the range 182–192 g eq−1 and a viscosity in the range 11,000–14,000 mPa·s at 25 °C; and tri-glycidyl para-amino phenol (TGAP), Araldite MY5010 (Huntsman Advanced Materials), another commercial resin with an epoxy equivalent in the range 95–106 g eq−1 and a much lower viscosity, in the range 550–850 mPa·s at 25 °C. Each resin was mixed with a small mass fraction of a commercial organically modified clay, montmorillonite (MMT), Nanomer I.30E (Nanocor Inc.), with a cation exchange capacity of 0.92 meq g−1 and in which the organic modifier is octadecylammonium.
For the DGEBA epoxy, two different curing agents were used separately, each in a stoichiometric ratio with the resin: a polyoxypropylene diamine, Jeffamine D-230 (Huntsman Corporation); and a commercial hyperbranched polymer (HBP), an –NH2 terminated poly(ethyleneimine), Lupasol PR8515 (BASF), which has a viscosity at 20 °C in the range 10,000–20,000 mPa·s and an average molecular mass of 2,000. For the TGAP epoxy resin, the crosslinking agent was diaminodiphenyl sulphone (DDS), Aradur 976-1 (Sigma Aldrich), and was used in a ratio with excess epoxy (1:0.85 molar TGAP:DDS) [19, 20].
To prepare the nanocomposites, firstly premixes of resin and clay were prepared, usually with small clay contents of 2 and 5 mass%, but occasionally with much larger contents of 10 and 20 mass%. For the DGEBA epoxy resin, the method of preparation consisted of three stages: initially the nanoclay and the resin were placed in a vessel with a magnetic stirrer (Jenway 1103) on a hotplate at 40–50 °C for 30 min, followed by mixing in an ultrasonic bath (Branson 3510) for 3 h at 45 °C, in order to improve the dispersion of the clay in the resin. Finally, the samples were drastically dispersed using a sonicator (Branson S450) in pulse mode and at 30 % amplitude for a total of 9.0 min, with a program of six separate steps of 1.5 min each, in which the sample was sonicated in three pulses of 30 s duration, with 30 s between them. The maximum temperature was limited to 45 °C by immersing the container, in which the sonication was taking place, in an ice/salt bath and allowing up to 1 h between the 1.5 min steps for the temperature to reduce sufficiently. For the TGAP epoxy resin, for which the viscosity is much lower and hence allows easier dispersion of the clay in the resin, the premix of resin and clay was made simply by mechanical mixing.
Once the resin/clay premixes had been prepared, the curing agent was added, in a stoichiometric ratio for the DGEBA and in a ratio with excess epoxy for the TGAP. The curing agent was mixed in by hand in small quantities on a watch glass, at room temperature and rapidly for both the Jeffamine and Lupasol, and for 5–7 min on a hotplate at 80 °C for the DDS. These samples were then immediately degassed under vacuum at room temperature, ready for the thermal analysis.
Thermal analysis
The cure kinetics of the prepared samples was monitored by DSC, in either non-isothermal scans at 2, 5, 10, 15, and 20 K min−1 and isothermal scans at temperatures between 50 and 90 °C for the DGEBA epoxy, or in isothermal experiments at temperatures between 120 and 180 °C for the TGAP epoxy. The equipment used was a Mettler-Toledo DSC 821e differential scanning calorimeter equipped with a sample robot and Haake EK90/MT intracooler. All DSC curing experiments were performed with a dry nitrogen gas flow of 50 mL min−1. The data evaluation was performed with the STARe software. The DSC was calibrated for both heat flow and temperature using indium. For all the experiments, a small sample of about 8-10 mg was weighed into an aluminium pan, sealed, and immediately inserted into the DSC furnace, which was previously heated either to the start temperature for non-isothermal scans or to the curing temperature for isothermal experiments, whereupon the curing experiment was immediately started.
Nanostructural characterisation
The nanostructure of the cured nanocomposites was examined by SAXS and TEM. For SAXS, cured bulk samples were converted to powder in a ball mill (Retsch model MM 400) using 20-mm-diameter steel balls and a frequency of 20 Hz for a period of 4 min. A Bruker D8 Advanced diffractometer was used to obtain the scattering diagram, measurements being taken in a range of 2θ = 1°–8° with copper Kα radiation, the scans being made with steps in 2θ of 0.02° and with a time of 10 s for each step.
TEM was carried out with a Jeol Jem-2010 High-Resolution electron microscope, with an accelerating voltage of 200 kV. Samples were prepared by ultramicrotomy of the bulk cured nanocomposites, to give a section of about 50 nm thickness.
Results and discussion
Nanocomposites with DGEBA and Jeffamine
These nanocomposites of DGEBA epoxy resin and clay, with polyoxypropylene diamine as the crosslinking agent, were prepared with 10 and 20 mass% MMT in the manner described above, and were then cured non-isothermally in the DSC. A typical result is shown in Fig. 1 for these two nanocomposites together with the same epoxy-amine system without any clay [16]. Several features are immediately apparent. First, it is clear that the initial rise in the heat flow, as well as the temperature at which the peak heat flow occurs are both advanced by the presence of the organically modified clay, which acts as a catalyst for the curing reaction.
DSC cure curves at 2.5 K min−1 heating rate for the DGEBA–Jeffamine system with the following clay contents: without clay (filled diamond); 10 mass% (filled triangle); 20 mass% (asterisk). The full lines represent the fit of the autocatalytic model. Exothermic direction is upward, and specific heat flow is referred to the total sample mass, including clay
It is also evident that the cure curves for the two nanocomposite systems are not symmetrical, there being a significant shoulder which appears on the high-temperature flank of each curve. This can be more clearly seen by fitting the cure reaction with an autocatalytic model [16], the fit being indicated in Fig. 1 by the full lines. Whereas an excellent fit is obtained for the system without clay, both of the nanocomposites show large deviations in the region after the peak, and more so the higher is the clay content.
This is clearly an effect of the clay, which catalyses a homopolymerisation reaction of the epoxy resin that is intercalated within the clay galleries [16, 21]. This cationic polymerisation process is initiated by the alkylammonium ion of the organically modified MMT, which reacts with an epoxy group to produce an oxonium ion. The propagation takes place by the reaction of the oxonium ion with other epoxy groups, and results in a highly branched and/or crosslinked polyether [22]. This reaction, which takes place even in epoxy/clay mixtures before the addition of the curing agent and is referred to as pre-conditioning [23], can be beneficial for the exfoliation process as it promotes the reaction within the clay galleries [21]. On the other hand, the homopolymerisation reaction within the clay galleries means that the bulk crosslinking reaction will take place in non-stoichiometric conditions. The effect of this is not the subject of the present paper, but has been discussed by other authors [24, 25].
In respect of exfoliation of the DGEBA–Jeffamine nanocomposite, the appearance of the shoulders seen in Fig. 1 at temperatures higher than that of the peak is not beneficial in the present circumstances. This is because the intra-gallery homopolymerisation reaction is occurring after the major part of the bulk crosslinking reaction has taken place; in other words, the effect of the intra-gallery reaction, which should result in a separation of the clay layers leading to exfoliation, is inhibited by the presence of a rigid surrounding network. As a consequence, very little exfoliation actually takes place in these nanocomposites. In fact, rather than exfoliating the clay layers, the volume contraction that occurs in the bulk extra-gallery regions during the crosslinking reaction actually gives rise to a reduction in the d-spacing of the clay layers [16]. This can be seen in the SAXS diffraction pattern shown in Fig. 2, for a nanocomposite with only 2 mass% clay, where a strong scattering peak is observed at 2θ = 6°, approximately, corresponding to a d-spacing of about 1.4 nm, which is less than that of the epoxy-intercalated clay (2.1 nm) before the addition of the diamine.
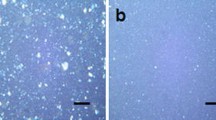
Further confirmation of this nanostructure is seen in the TEM micrograph of Fig. 3, which shows a large amount of layer stacking in the cured nanocomposite and very little evidence of exfoliation.
It is evident, therefore, that the nanocomposites prepared with DGEBA epoxy resin and cured with Jeffamine, and containing either 2, 10, or 20 mass% clay, do not have an exfoliated nanostructure. This is clear from the SAXS and TEM characterisations, but it can also be deduced from the DSC cure curves, where the extra-gallery crosslinking reaction occurs before the intra-gallery reaction, thus inhibiting the separation of the clay layers. In the following example, a different situation will be demonstrated.
Nanocomposites with DGEBA and HBP (Lupasol)
When the same epoxy resin/clay mixtures as used above with Jeffamine as the curing agent are cured instead with the hyperbranched polymer, Lupasol, the non-isothermal cure reaction observed by DSC is significantly different. Figure 4 shows the cure curves for four samples, with clay contents of 0, 2, 5, and 10 mass%, all cured non-isothermally at 20 K min−1. In contrast to the cure curves for the DGEBA–Jeffamine system shown in Fig. 1, the cure curves for the DGEBA–Lupasol system are symmetric, and do not display the shoulder on the high-temperature flank of the exotherm.
DSC cure curves at 20 K min−1 heating rate for DGEBA–Lupasol system with the following clay contents: without clay (filled diamond); 2 mass% (filled circle); 5 mass% (times symbol); 10 mass% (filled triangle). Exothermic direction is upward, and specific heat flow is referred to the total sample mass, including clay
There are still both intra-gallery homopolymerisation and extra-gallery crosslinking reactions occurring, just as there were in the DGEBA–Jeffamine nanocomposite system, but the implication of the results shown in Fig. 4 is that the intra-gallery reaction is now hidden underneath the crosslinking reaction of the DGEBA and Lupasol. This means that the two reactions are now occurring at least concurrently, and hence partial exfoliation of the clay layers as a consequence of the intra-gallery reaction is able to take place to some extent before the bulk crosslinking inhibits any further nanostructural development. This would be expected to result in the disappearance of the strong diffraction peaks seen in the DGEBA–Jeffamine nanocomposites discussed above, and indeed this is what is observed. Figure 5 shows the SAXS diffraction pattern for this cured nanocomposite, where it can be seen that, in contrast to that shown in Fig. 2 for the DGEBA–Jeffamine nanocomposite, there is no scattering peak within the range of low angles shown here.
It would be anticipated, therefore, that the TEM micrographs would confirm this by showing a greater degree of exfoliation for these DGEBA–Lupasol nanocomposites, and again this is indeed the case. Figure 6 shows the comparison between the TEM micrographs for nanocomposites containing 2 mass% MMT and cured with Jeffamine (Fig. 6a) and with Lupasol (Fig. 6b). Here, it can be seen that the Jeffamine cured nanocomposite continues to display considerable layer stacking, with little evidence of any exfoliation, whereas the Lupasol cured nanocomposite retains only a few layers still in register, with a d-spacing of about 6–7 nm, but more importantly shows a significant proportion of clay layers which are widely separated and randomly dispersed, in other words with a significant degree of exfoliation.
Nanocomposites with TGAP and DDS
A quite different situation prevails when the nanocomposites are fabricated from resin/clay mixtures prepared using the trifunctional epoxy resin, TGAP, and cured isothermally with DDS [26]. The cure curves for this system are illustrated in Fig. 7 for three different isothermal cure temperatures, where it can be seen that there are two quite distinct reactions: the first, very rapid, corresponds to the intra-gallery homopolymerisation of the epoxy, catalysed by the organically modified MMT, while the second rather broad bell-shaped peak results from the crosslinking reaction of the TGAP with the DDS. The assignment of the rapid initial reaction to the homopolymerisation of the epoxy is confirmed by its absence in the isothermal cure of the same system in which the TGAP and MMT have previously been pre-conditioned, by storage for a period of time at a selected temperature, during which process there is a spontaneous homopolymerisation reaction of the epoxy resin [11].
These two reactions evident in Fig. 7 can be deconvoluted into two separate peaks, from which it is found that the magnitude of the first, intra-gallery, peak increases with increasing isothermal cure temperature. Similar results are found when the cure kinetics is studied at a fixed isothermal cure temperature and the effect of the MMT concentration is examined [26]: here, the magnitude of the first peak increases as the MMT content increases. The implications of these observations are that in this TGAP/MMT/DDS nanocomposite system the intra-gallery homopolymerisation reaction occurs before the bulk, extra-gallery, crosslinking reaction, and to a greater extent the higher is the isothermal cure temperature. The occurrence of the intra-gallery reaction now before, rather than after (as in the case of the DGEBA–Jeffamine system) or concurrently with (as in the case of the DGEBA–Lupasol system), the extra-gallery crosslinking reaction, would be expected to result in an enhanced degree of exfoliation of this TGAP/MMT/DDS nanocomposite system in comparison with the other systems, and this is indeed supported by the nanostructural characterisation of the cured nanocomposite by SAXS and TEM.
This is illustrated in Fig. 8, for the TGAP/MMT/DDS system with 5 mass% of clay, cured isothermally at 180 °C, which shows that there are no diffraction peaks at low scattering angles for this cured nanocomposite. The same result was obtained for the lower cure temperatures of 150 and 120 °C, suggesting a good degree of exfoliation in this nanocomposite system.
This was confirmed by TEM characterisation of the same nanocomposite with 5 mass% MMT, cured isothermally at 180 °C, as shown by the micrograph in Fig. 9. Here, it can be seen that the clay layers are now no longer in register, being distributed with rather random orientations, and that their separation is typically greater than about 10 nm. The degree of exfoliation for this system is, therefore, significantly better than for either of the other two systems described above, and this conclusion could be anticipated already from the DSC cure kinetics data shown in Fig. 7.
Conclusions
Three examples have been shown of how the DSC cure kinetics of epoxy polymer layered silicate nanocomposites can complement the structural characterisation methods of SAXS and TEM for the identification of the degree of exfoliation of the cured nanocomposite.
-
(i)
For the DGEBA/MMT/Jeffamine system, the existence of a pronounced shoulder on the high-temperature flank of the non-isothermal DSC cure curve indicates that the intra-gallery homopolymerisation reaction is occurring too late, after the extra-gallery crosslinking reaction, and as a consequence the exfoliation is inhibited. This is confirmed by both SAXS and TEM, which show a large proportion of ordered clay layers, even with a d-spacing less than that of the resin-intercalated clay.
-
(ii)
For the DGEBA/MMT/HBP system, the shoulder on the high-temperature flank of the non-isothermal cure curve is no longer present, suggesting that the intra-gallery reaction takes place concurrently with the crosslinking reaction. This implies that there is a greater opportunity for exfoliation to take place, and this is again confirmed by both SAXS, for which the scattering peak corresponding to very small d-spacings no longer appears, and TEM, which shows significantly larger d-spacings and considerably less parallel stacking of the clay layers.
-
(iii)
For the TGAP/MMT/DDS system, the intra-gallery reaction in isothermal cure occurs before the extra-gallery crosslinking reaction, and hence implies that significantly more exfoliation should occur. This is once again confirmed by SAXS, which shows no scattering peaks, and by TEM, which shows rather randomly distributed clay layers with essentially no layer stacking.
The DSC cure curves, therefore, provide evidence about the likelihood of exfoliation being attained in the cured nanocomposites, and thus DSC serves as a useful tool for such studies, in particular for eliminating the need to pursue the nanostructural characterisation of samples which are unlikely to be significantly exfoliated.
References
Alexandre M, Dubois P. Polymer-layered silicate nanocomposites: preparation, properties and uses of a new class of materials. Mater Sci Eng. 2000;28:1–63.
Becker O, Simon GP. Epoxy layered silicate nanocomposites. Adv Polym Sci. 2005;179:29–82.
Azeez AA, Rhee KY, Park SJ, Hui D. Epoxy clay nanocomposites–processing, properties and applications: a review. Composites part B. 2013;45:308–20.
Brown JM, Curliss D, Vaia RA. Thermoset-layered silicate nanocomposites. Quarternary ammonium montmorillonite with primary diamine cured epoxies. Chem Mater. 2000;12:3376–84.
Chen C, Curliss D. Preparation, characterization, and nanostructural evolution of epoxy nanocomposites. J Appl Polym Sci. 2003;90:2276–87.
Becker O, Cheng Y-B, Varley RJ, Simon GP. Layered silicate nanocomposites based on various high-functionality epoxy resins: the influence of cure temperature on morphology, mechanical properties, and free volume. Macromolecules. 2003;36:1616–25.
Velmurugan R, Mohan TP. Room temperature processing of epoxy-clay nanocomposites. J Mater Sci. 2004;39:7333–9.
Mohan TP, Ramesh Kumar M, Velmurugan R. Rheology and curing characteristics of epoxy-clay nanocomposites. Polym Int. 2005;54:1653–9.
Lipinska M, Hutchinson JM. Elastomeric epoxy nanocomposites: nanostructure and properties. Comp Sci Tech. 2012;72:640–6.
Román F, Calventus Y, Colomer P, Hutchinson JM. Identification of nanostructure development in epoxy polymer layered silicate nanocomposites from the interpretation of differential scanning calorimetry and dielectric spectroscopy. Thermochim Acta. 2012;541:76–85.
Hutchinson JM, Shiravand F, Calventus Y. Intra- and extra-gallery reactions in tri-functional epoxy polymer layered silicate nanocomposites. J Appl Polym Sci. 2012;128:2961–70.
Lan T, Kaviratna PD, Pinnavaia TJ. Mechanism of clay tactoid exfoliation in epoxy-clay nanocomposites. Chem Mater. 1995;7:2144–50.
Park JH, Jana SC. Mechanism of exfoliation of nanoclay particles in epoxy-clay nanocomposites. Macromolecules. 2003;36:2758–68.
Chin I-J, Thurn-Albrecht T, Kim H-C, Russell TP, Wang J. On exfoliation of montmorillonite in epoxy. Polymer. 2001;42:5947–52.
Kornmann X, Lindberg H, Berglund LA. Synthesis of epoxy-clay nanocomposites. Influence of the nature of the curing agent on structure. Polymer. 2001;42:4493–9.
Montserrat S, Román F, Hutchinson JM, Campos L. Analysis of the cure of epoxy based layered silicate nanocomposites: reaction kinetics and nanostructure development. J Appl Polym Sci. 2008;108:923–38.
Román F, Montserrat S, Hutchinson JM. On the effect of montmorillonite in the curing reaction of epoxy nanocomposites. J Therm Anal Calorim. 2007;87:113–8.
Leszczynska A, Pielichowski K. Application of thermal analysis methods for characterization of polymer/montmorillonite nanocomposites. J Therm Anal Calorim. 2008;93:677–87.
Varley RJ, Hodgkin JH, Hawthorne DG, Simon GP. Toughening of a trifunctional epoxy system. 2. Thermal characterization of epoxy/amine cure. J Appl Polym Sci. 1996;60:2251–63.
Hutchinson JM, Shiravand F, Calventus Y, Fraga I. Isothermal and non-isothermal cure of a tri-functional epoxy resin (TGAP): a stochastic TMDSC study. Thermochim Acta. 2012;529:14–21.
Pustkova P, Hutchinson JM, Román F, Montserrat S. Homopolymerization effects in polymer layered silicate nanocomposites based upon epoxy resin: implications for exfoliation. J Appl Polym Sci. 2009;114:1040–7.
Hutchinson JM, Montserrat S, Román F, Cortés P, Campos L. Intercalation of epoxy resin in organically modified montmorillonite. J Appl Polym Sci. 2006;102:3751–63.
Benson Tolle T, Anderson DP. The role of preconditioning on morphology development in layered silicate thermoset nanocomposites. J Appl Polym Sci. 2004;91:89–100.
Gude MR, Prolongo SG, Urena A. Effect of the epoxy/amine stoichiometry on the properties of carbon nanotube/epoxy composites. J Therm Anal Calorim. 2012;108:717–23.
Del Cid MAG, Prolongo MG, Salom C, Arribas C, Sanchez-Cabezudo M, Masegosa RM. The effect of stoichiometry on curing and properties of epoxy-clay nanocomposites. J Therm Anal Calorim. 2012;108:741–9.
Shiravand F, Hutchinson JM, Calventus Y. Influence of the isothermal cure temperature on the nanostructure and thermal properties of an epoxy layered silicate nanocomposite. Polym Eng Sci. 2014;54:51–8.
Acknowledgements
The authors are grateful to the Dow Chemical Company for the DGEBA epoxy resin, to the Huntsman Corporation for the TGAP epoxy resin and curing agent, and to Nanocor Inc. for the organically modified clay. This work was supported by the Ministerio de Ciencia e Innovación (MICINN), Project MAT2011-27039-C03-03. FS is grateful for a Grant from the Agència de Gestió d’Ajuts Universitaris i de Recerca (AGAUR), FI-DGR 2011.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shiravand, F., Fraga, I., Cortés, P. et al. Thermal analysis of polymer layered silicate nanocomposites. J Therm Anal Calorim 118, 723–729 (2014). https://doi.org/10.1007/s10973-014-3709-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-014-3709-3