Abstract
Combustion behavior of Indian lignite sample blended with rice husk chars (prepared at low temperature) has been examined in this study through simultaneous differential scanning calorimetry (DSC) and thermogravimetric analysis (TG) technique. Range of inputs obtainable by characteristic TG–DSC parameters has been utilized to arrive at important conclusions and observations in respect of ideal selection of blend proportion, proper utilization of the blend combination, etc. Deviations of experimental mass loss pattern (TG) and of rate curve (DTG) from corresponding expected theoretically calculated pattern have also been noted for different blends to examine possible advantageous or disadvantageous effects. As per the observations recorded, use of biomass char in blends (with lignite) was found to be very much beneficial and its proportion in the blends may be restricted to a level of 40 % by mass to extract maximum benefits in burning performance. This paper also focuses specific advantages of use of rice husk char in place of raw rice husk for cocombustion applications. Moreover, the importance of heat release pattern to assess compatibility of a fuel mix with existing boiler design and also to workout fresh boiler design for cocombustion application has been discussed in this paper.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cocombustion of biomass and coal/lignite for production of energy is a promising option under the present energy scenario all over the globe. The world is facing technical challenges with cocombustion of biomass and coal/lignite in boilers designed for pulverized coal/lignite combustion. The combustion of coal/lignite in the presence of biomass is of great interest in respect of control of CO2 emissions as biomass is considered to be carbon neutral. Additional use of biomass can reduce emission of NO x and SO x . The technical issues associated with such cofiring include, fuel supply mechanism, performance, handling and storage challenges, corrosion and depositional issues, pollutant emissions, carbon burn out, and overall economics. In spite of the above challenges cocombustion is possibly best energy option for the future power generation of India, where plenty of different kinds of biomass is available. Motivations to reduce CO2 emissions, particularly in countries like India again strengthen the foundation of the rationality for cocombustion. Cocombustion has been demonstrated in various levels and scales with different boiler systems, fuel type, and categories of biomass [1–6]. Biomass fuels have sometimes been reported to have peculiar combustion features particularly when they are subjected to thermal shock [7]. As compared to coal, biomass is bulkier with higher content of volatile matter and moisture and lower content of energy [8]. High moisture content and low energy content of raw biomass are major shortcomings for use of those raw products as cofuel, and therefore, pretreatment of raw biomass may be necessary before it is blended with coal/lignite. As a consequence, use of biomass chars instead of raw biomass may be a preferred option in cocombustion with fossil fuel [9]. Several researchers have been reported that biomass chars obtained after partial devolatilization are more reactive. Chars originated from biomass are generally porous with highly disordered carbon structure and belong to the class of highly reactive carbon materials. The char porosity promotes accessibility of the reactive gas (oxygen) at the active sites resulting in very good combustion reactivity [10–12].
The application of cocombustion technologies involving biomass and coal for power generation requires a proper understanding of the chemical and thermal properties as well as reaction kinetics of coal/biomass blends. The information pertinent to thermal events and kinetics plays an important role in the efficient design, operation, and modeling of cofired boilers.
In India there is growing need of expansion of lignite-based power plant, especially in Southern and Western region of the country. Indigenous lignite production will not be sufficient to cater the future need of power supply through lignite-based power plant. Hence, significant import of lignite to bridge demand supply gap may be necessary. Considering economics and drives toward clean energy development program, future power generation practices through lignite-based power plants may utilize biomass or biomass derived char as cofuel. Emerging concept of cofuel application in lignite-based power plant demand critical examination of combustion performance of blends comprised of lignite and biomass char before utilizing the blends in boiler.
Thermogravimetric analysis (TG), which provides a measurement of mass loss of the sample as a function of time or temperature, is considered to be an important means to obtain basic combustion features of blends, i.e., to investigate thermal events and kinetics during combustion of coal or coal/biomass mixtures. Characteristic combustion parameters obtained from TG curve guides the selection of blend combination and optimum proportion. Cocombustion studies in TG, which revealed basic combustion behavior of coal–biomass or lignite–biomass blends, have been reported by several researchers [10, 13–26], but those with coal-biomass char blends are very rare, which was also attempted in one of our earlier work [9]. Interestingly enough, effectiveness of biomass/biochar combination in cocombustion has been described in one recent publication [27]. All these indicate that the applicability of bio-char as cofuel is quite promising in present context.
Under the above scenario cocombustion of low rank coal (lignite) and different types of biomass needs to be studied, particularly in Indian context, with an objective of maximizing biomass utilization (renewable option) and saving lignite (a fossil fuel). Therefore, as per the gravity of the need (in fact cocombustion studies in general have been included in clean coal technology program under 11th and 12th 5-year plan) the present study was undertaken at our Institute which is a premier coal research Institute under Ministry of Science and Technology, Govt. of India. In the present study, a blend combination comprised of lignite and rice husk char (prepared at 300 and 450 °C) was selected, and attempts were made to evaluate combustion behavior of blend samples of varying compositions by using simultaneous thermal analyzer (DSC–TG). In this study, theoretical rate curves for the blends were evaluated (based on DTG profiles of lignite and respective biomass chars) to see the deviations of the experimentally observed rate curves from the corresponding theoretical ones. Similar comparisons between theoretical and experimental TG profile have been made for the blend samples, and comparisons were reassessed on combustible basis. On the other hand it is important to know the resultant heat release pattern of the blend samples because boiler performance is very much dependent on the heat release rate of the fuels at different locations. Therefore, some observations on DSC curves have also been focused to reveal heat release pattern of blend samples with reference to blend components (parent coal and biomass).
Experimental
Coal selection and sample preparation
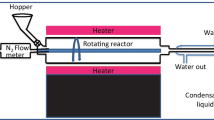
In this study one lignite sample (Neyveli Lignite Corporation, India) and a typical rice husk sample were chosen as constituents. The lignite sample was crushed to −3 mm size at first and then crushed to −212 μ size. Rice husk (RH) sample was air dried and equilibrated under laboratory condition to reduce moisture content. Rice husk sample was pyrolyzed at 300 and 450 °C. The char preparation was carried out in a specially designed muffle furnace where nitrogen atmosphere was maintained with a flow rate of 100 mL min−1. The samples were heated at a rate of 20 °C min−1 to the final pyrolysis temperature (300/450 °C) with 1 h residence time to produce two different char samples.
Char samples and lignite sample were first pulverized to −212 μ size. Then portions of pulverized rice husk chars were blended with pulverized lignite to prepare different binary blends of varying composition, where proportion of rice husk char varied from 10 to 50 %. The blends containing 10, 20, 30, 40, and 50 % of respective rice husk char blends have been designated as LRH1, LRH2, LRH3, LRH4, and LRH5. Two char samples as well as all the blend samples were further ground to −75 μ size for the combustion studies.
Chemical analyses
Proximate and ultimate analyses of samples were done using standard procedures i.e., IS: 1350—Part-I: 1984, Part III: 1969, Part IV/1:1974, Part IV/2: 1975 and ASTM E871, D1102, E872. High heating value (HHV) of lignite was determined as per IS: 1350 (Part 2): 1970. High heating value (HHV) of rice husk sample was also determined by using formula \( {\text{HHV}} = \;\left( {{{ 3 3. 5 {\text{C}} + 1 4 2. 3 {\text{H}} - 1 5. 4 {\text{O}} - 1 4. 5 {\text{N}}} \mathord{\left/ {\vphantom {{ 3 3. 5 {\text{C}} + 1 4 2. 3 {\text{H}} - 1 5. 4 {\text{O}} - 1 4. 5 {\text{N}}} {100}}} \right. \kern-0pt} {100}}} \right) \) [28, 29] where C, H, N, and O are in mass% dry ash free basis. The chemical data of the samples are shown in Table 1. As slagging/fouling phenomena are very common in cofired boilers causing deterioration of boiler performance, ash analyses of the samples were done as per standard procedure (IS: 1355:1984) to derive slagging and fouling index of the samples [30]. Ash composition, slagging, and fouling index of the samples are presented in Table 2.
Thermal analysis: DSC/TG/DTG
Combustion behavior of the samples was determined by using simultaneous thermal analyzer, model STA 409C (NETZSCH, Germany) at a heating rate of 10 °C min−1 in air flow rate of 50 mL min−1 with sample mass of 10 ± 1 mg. The sample mass loss was recorded continuously under dynamic conditions as a function of time or temperature to produce combustion profiles. The combustion profiles were analyzed to determine the various thermal parameters such as DSC peak temperature, DTG peak temperatures, and burn out temperature (BOT). DTG peak temperature is the temperature where rate of mass loss is maximum and BOT is the temperature at which the rate of combustion diminishes to 1 % min−1 at the end of the major combustion region. DSC peak temperature denotes the peak of combustion process in terms of heat flow pattern which is an important heat release characteristics.
Results and discussion
General fuel characteristics of all the fuels under consideration (i.e., lignite, raw rice husk, and rice husk char samples) have been reported in Table 1. It is the same rice husk, which was used in our earlier study [9]. The basic reasons behind the selection of rice husk char as a blend component instead of raw rice husk were explained in our published work [9]. Lignite–rice husk char combination was appeared to be very much potential for cofuel application, because improvement of heat input (i.e., heat input to the boiler) from the biomass component or heat intensity of biomass (up to the level comparable with lignite) and lowering of volatile matter level to be compatible with lignite are possible with the use of low temperature char of rice husk. All these have been reflected in Table 1. Fuel ratio of rice husk char prepared at 300 °C (RH 300) is 1.22 (Table 1) which may be considered as very good fuel ratio to raise expectation level toward obtaining good combustion performance. It is to be noted here that desired fuel ratio in Indian context is <2.5 for satisfactory combustion performance in pulverized fuel firing [9], which is considered to be the limiting specification in some other countries also, e.g., in Japan [9]. As such, fuel ratio is an important parameter for controlling the burning performance in a pulverized fuel firing system. It is also known that for pulverized firing system, VM level in the range of 22–35 % [9] is generally preferred. As a matter of fact, consideration of fuel ratio and resultant VM content of the fuel mix are important for combustion practices. From the values of these two parameters (Table 1), it appears that cocombustion of lignite-rice husk char combination should work well in pulverized fuel firing systems. Ash compositions for the samples are very important for such cocombustion process, and all these compositions give significant insight about the risk of slagging and fouling. For examining the above mentioned risk related to performance failure, slagging index and fouling index were computed for parent fuels as well as blends. Proneness to slagging and fouling are said to be low if the values of slagging index and fouling index are less than 0.6 and 0.2, respectively [30]. From the estimated values of slagging index and fouling index (Table 2), it may be noted that in all cases values are well within the aforesaid prescribed limit indicating low propensity of boiler slagging as well as boiler fouling.
Significant combustion parameters obtained through TG–DSC curves have been presented in Table 3. In order to investigate whether interaction occurred between the components of blends, theoretical rate curves were calculated based on the same temperature history of the two component fuels [27]. The curves represented the weighted mean of the individual component’s behavior. Theoretical rate of mass loss of the blend at any instant of time or temperature may be represented as
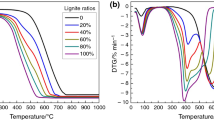
where (dm/dt)lignite, (dm/dt)biomass char are the normalized rates of mass loss, as found from the individual experiments, and x 1, x 2 are the mass fraction of lignite and biomass chars in the blends, respectively. The theoretical (calculated) and experimental rate curves for the representative blends of lignite-rice husk char are shown in Figs. 1, 2. Experimental rate curves exhibited by the blends of rice husk char prepared at 300 °C resemble theoretical curve at lower proportion of char content (up to 20 %). At higher proportion of 300 °C char content the experimental curve shifts toward right to theoretical curve. As such Fig. 1 indicates that the interactive effect becomes prominent at higher proportion of 300 °C char and such interactions caused lowering of rate values throughout the temperature range as compared to corresponding theoretical rate values. Therefore, use of 300 °C char at higher proportion caused little lowering of reactivity (antisynergistic effect) and no reactivity improvement over weighted mean value was practically noticed. Moreover, the deviations of experimental rate curves from corresponding theoretical rate curves have not been found to be significant on overall basis (Fig. 1) and it should not have any serious effect on the burn out performance of the blends. Nature of shifting of experimental curve with reference to calculated curve incase of 450 °C char blends (Fig. 2) is similar to those of above mentioned 300 °C char blends with higher char content. Appearance of second peak (hump) after major DTG peak in blends containing 40 and 50 % 450 °C char reflect step wise burning of blend components. However, interactive effects between the components are prominent from delayed appearance of DTG peaks in blend samples as compared to respective peak of theoretical curve (Fig. 2). In case of 450 °C char also, the rate curves are not significantly away from calculated curves. Positive and negative interactive interactions have been noticed by several researchers in case of coal blends [31–34] as well as coal-biomass blends [10, 19, 20, 35–37] leading to additive, synergistic, and antisynergistic effects. For coal-biomass blends, synergistic effects were mostly observed by the researchers [20, 35–37]. However, for coal-wood char combination in cocombustion process, the temperature zone of wood char burning was found by Kastanaki and Vamvuka [10] to be clearly differentiated from coal and this was identified as the possible cause of inconsiderable interaction between the constitutive parts. In the above study [10] authors have also observed the synchronized burning of biomass char and lignite during cocombustion of both the components leading to positive interaction.
In the present investigation, it may be noted from Table 3 that reactivity parameters (DTG peak temperature and BOT) of both the 300 °C char and 450 °C char samples are lower than those of lignite samples. During cofiring, the relatively inert above char samples tend to be combusted at the later stage leading to antisynergistic effect. The step wise burning of blend components could be ultimately detected through the appearance of distinct double DTG peak in case of blends containing 40 and 50 % 450 °C char. Therefore, antisynergistic effect in most of the char blends, although appeared as minor, is practically due to delayed burning of char components. In general, such findings have immense importance for deciding blend combination as well as blend composition in case of cofired boiler. Because, synchronization of burning characteristics of two blend components is very important for boiler operation. On the other hand, delay in release of heat from either of the components may lengthen the overall burn out time, which may or may not be beneficial depending on the basic design of the combustor.
In this paper theoretical BOT of the blend samples were derived directly from theoretical rate curves. These theoretical BOT and experimentally determined BOT have been plotted against biomass char content in the blend and represented in Fig. 3a, b. The figures depict that burn out temperature of blend samples is generally higher than theoretically derived BOT values particularly for lower proportion of biomass chars in the blends. This implies blends containing lower proportion of biomass char may take little more time to burn out with respect to predictions made through theoretical curves.
Likewise, rate curves (Figs. 1, 2), mass loss pattern of blends in TG experiments have been compared with calculated mass loss pattern based on TG data points of individual blend components (Fig. 4a, b). Data points from 200 to 600 °C have been considered for such comparison, as the entire combustion zone for all the samples are covered in this temperature range. For all the blend samples, it has been observed that experimental curve always deviate from respective theoretical (calculated) one. An example of such comparison between experimental and calculated curve resulting in substantial deviation has been shown in Fig. 4a. This indicates that loss pattern of the blends fail to fulfill additivity criteria. Another point is that combustibles are basically organic part of the fuel mix and it is interesting to note that the deviation from calculated curve is diminished if the entire results are recalculated on combustible basis (Fig. 4b), i.e., the situation will be closer to additivity. Although such closeness to theoretical (calculated curve) does not have specific implications in respect of burning performance, loss patterns may be relooked on combustible basis for understanding of combustion characteristics of blend from a different angle.
Heat flow patterns of blend components (lignite and biomass char) and blend samples as evident from DSC curves are also very important to reveal heat release characteristics of blends during combustion. Those patterns of blends of 300 °C chars have been compared with those of respective blend components in Fig. 5 under the same reference frame. It may be noticed that heat flow patterns of all the blend samples are close to that of lignite sample and far apart from that of char sample. Therefore, it appears that on overall basis heat flow pattern of the blend sample is influenced by lignite. Moreover, no peak separation occurred in DSC patterns of the blends (Fig. 5), which reflect compatibility of the blend components (rice husk char and lignite) with respect to release of heat. In case of lignite-450 °C char blends, overall heat flow pattern of all the blend samples was also shifted to that of lignite as compared to mean position indicating major influence of lignite in cocombustion. Therefore, rice husk char formed by low temperature pyrolysis is likely to be a good choice for cofiring application in pulverized fuel firing system.
From the partial area of DSC curve, estimation of percentage of heat release at different time or temperature is possible. Conversely, evaluation of time or temperature for different level of heat release (25, 50, 75, 90 %) is also possible and the scenario have been depicted in Table 4 and Fig. 6a, b. These levels have been chosen judiciously with an objective of comparing relative time span (as temperature in Table 4 and Fig. 6a, b is proportional to time for all the experiments conducted at constant heating rate) to achieve the aforesaid levels of heat release. Heat release to the extent of 50 % is very much important because after this point the combustion is essentially char burning, which is the rate determining step. 25 and 75 % levels are the mid way to arrive 50 % and complete burn out level, respectively. 90 % heat release level signifies terminal phase of the combustion and also suitable for comparison purpose.
It appears from Table 4 and Fig. 6a, b that the time or temperature of heat release at initial levels (i.e., at lower levels of heat release) of blend samples is close to that of lignite, whereas at higher level of heat release, corresponding time/temperatures in case of blends is close to respective time/temperature of biomass char sample. Therefore, it may be inferred here that although the entire process of heat release is principally governed by lignite, heat release at final stages is governed by biomass char samples. This shows inertness of the residual char product after 50 % combustion is over. It can be inferred that combustion heat release of the blend samples is shifted toward left to the mean position showing improvement of combustion features (Fig. 5), although delayed appearance of DTG peak temperature and BOT also occurs showing less reactivity at specific levels. All these information may be very much useful for designing a suitable combustor for cofuel application, particularly for estimating combustion space. On the other hand, for a given combustor such information may guide to select suitable blend combination and blend composition so as to meet specific design criteria linked with the required quantity of heat release at different locations along the length or height of the combustor.
Conclusions
Although interactive effects between the components are observed from DTG peak positions, on overall basis observed rate curves are not significantly away from calculated curves. It appears that blends containing low proportion of biomass char may take little more time to burn out with respect to predictions made through theoretical curves. Heat flow patterns of the blend samples during combustion (as it is evident from DSC plots) are principally governed by lignite. However, at the final stage of heat release biomass char plays the significant role, and not the lignite. In case of the blends, compatibility of the blend components (rice husk char and lignite) is also very much evident from the heat release pattern without any peak separation (DSC). Beside few observations like delayed burn out (indicating low reactivity at some specific phases), most of the observations suggested that low temperature biomass char should be a good choice for cofiring application in pulverized fuel firing system. Shifting of heat release patterns (DSC) of the blends toward the left to the respective mean positions clearly confirms advantage due to synergistic effects. Therefore, use of low temperature char with lignite may be advocated not only from view point of intensification of heat value in chars as compared to raw biomass, but also from synergistic effect point of view also. Information on heat release pattern with progress of burning process also appears to be valuable for assessing compatibility of certain fuel combination with the design of existing combustor and also for fresh design consideration of a combustor suitable for a selected fuel mix. As a whole, this study provides range of inputs/information for selection of suitable blend proportion/combination for cofiring of lignite and rice husk char in power industry.
References
Narayanan KV, Natarajan E. Experimental studies on cofiring of coal and biomass blends in India. Renew Energy. 2007;32:2548–58.
Williams A, Pourikashanian M, Jones JM. Combustion of pulverized coal and biomass. Progr Energy Combust Sci. 2001;27:587–610.
Sweeten JM, Annamalai K, Thien B, McDonald LA. Co-firing of coal and cattle feedlot biomass (FB) fuels. Part I. Feedlot biomass (cattle manure) fuel quality and characteristics. Fuel. 2003;82:1167–82.
Tillman DA. Cofiring benefits for coal and biomass. Biomass Bioenergy. 2000;9:363–4.
Lester E, Gong M, Thompson A. A method for source appiortionment in biomass/coal blends using thermogravimetric analysis. J Anal Appl Pyrol. 2007;80:111–7.
Sami M, Annamalai K, Wooldridge M. Cofiring of coal and biomass fuel blends. Prog Energy Combust Sci. 2001;27:171–214.
Biagini E, Cioni M, Tognotti L. Development and characterization of a lab scale entrained flow reactor for testing of biomass fuels. Fuel. 2005;84:1524–34.
Darvell LI, Jones JM, Gudka B, Baxter XC, Saddawi A, Williams A, Malmgren A. Combustion properties of some power station biomass fuels. Fuel. 2010;89:2881–90.
Sahu SG, Sarkar P, Chakraborty N, Adak AK. Thermogravimetric assessment of combustion characteristics of blends of a coal with different biomass chars. Fuel Process Technol. 2010;91:369–78.
Kastanaki E, Vamvuka D. A comparative reactivity and kinetic study on the combustion of coal–biomass char blends. Fuel. 2006;85:1186–93.
Backreedy RI, Jones JM, Pourkashanian M, Williams A. Burnout of pulverized coal and biomass chars. Fuel. 2003;82:2097–105.
Koufopanos CA, Maschio G, Lucchesi A. Kinetic modeling of the pyrolysis of biomass and biomass components. Canadian J Chem Eng. 1989;67:75–84.
DiBlasi C, Buoranno F, Branca C. Reactivities of some biomass chars in air. Carbon. 1999;37:1227–38.
Russel NV, Belley TJ, Mann CK, Gibbins JR, Williamson J. Development of TG measurements of intrinsic char combustion reactivity for industrial and research purposes. Fuel Process Technol. 1998;57:113–30.
Stenseng M, Zolin A, Cenni R, Frandsen F, Jensen A, Dam-Johansen K. Thermal analysis in combustion research. J Therm Anal Calorim. 2001;64:1325–34.
Henrich E, Burkle S, Meza-Renken ZI, Rumpel S. Combustion and gasification kinetics of pyrolysis chars from waste and biomass. J Anal Appl Pyrol. 1999;49:221–41.
Kastanaki E, Vamvuka D, Grammelis P, Kakaras E. Thermogravimetric studies of the behaviour of lignite–biomass blends during devolatilization. Fuel Process Technol. 2002;77–78:159–66.
Vamvuka D, Kakaras E, Kastanaki E, Grammelis P. Pyrolysis characteristics and kinetics of biomass residuals mixture with lignite. Fuel. 2003;82:1949–60.
Haykiri-Acma H, Yaman S. Effect of co-combustion on the burnout of lignite/biomass blends: a Turkish case study. Waste Manag. 2008;28:2077–84.
Haykiri-Acma H, Yaman S. Combinations of synergistic interactions and additive behavior during the co-oxidation of chars from lignite and biomass. Fuel Process Technol. 2008;89:176–82.
Haykiri-Acma H, Yaman S, Kucukbayrak S. Co-combustion of low rank coal/waste biomass blends using dry air of oxygen. Appl Therm Eng. 2013;50:251–9.
Zakaria Z, Mohd Ishak MA, Abdullah MF, Ismail K. Thermal decomposition study of coals, rice husk, rice husk char and their blends during pyrolysis and combustion via thermo gravimetric analysis. Int J Chem Technol. 2010;2(3):78–87.
Taş S, Yürüm Y. Co-firing of biomass with coals. Part 2. Thermogravimetric kinetic analysis of co-combustion of fir (Abies bornmulleriana) wood with Beypazari lignite. J Therm Anal Calorim. 2012;107:293–8.
Sun X, Yin S, Wang H, Li C, Zhang S. Effect of the addition of cornstalk to coal power/coal tar combustion. J Therm Anal Calorim. 2012;109:817–23.
Dumani AG, Taş S, Yürüm Y. Co-firing of biomass with coals. Part 1. Thermogravimetric kinetic analysis of combustion of fir (Abies bornmulleriana) wood. J Therm Anal Calorim. 2011;103:925–33.
Cioablă AE, Tordai T, Rotaru P, Socaciu M, Ionel I. Experimental approach of co-firing and anaerobic fermentation of biomass and coal, and their thermochemical properties. J Therm Anal Calorim. 2012;110:395–403.
Yi Q, Qi F, Cheng G, Zhang Y, Xiao B, Hu Z, Liu S, Cai H, Xu S. Thermogravimetric analysis of co-combustion of biomass and biochar. J Therm Anal Calorim. 2012; doi:10.1007/s10973-012-2744-1.
Demirbas A. Calculation of higher heating values of biomass fuels. Fuel. 1997;76:431–4.
Cordero T, Marquez F, Rodrigueza-Mirasol J, Rodriguez JJ. Predicting heating values of lignocellulosic and carbonaceous materials from proximate analysis. Fuel. 2011;80:1567–71.
Unsworth JF, Barratt DJ, Roberts PT. Combustion science and technology 19: coal quality and combustion performance. In: Unsworth JF, editor. Coal characterization. Amsterdam: Elsevier; 1991. p. 215–9.
Su S, Pohl JH, Holcombe D, Hart JA. Techniques to determine ignition, flame stability and burnout of blended coals in p.f. power station boilers. Progr Energy Combust Sci. 2001;27:75–98.
Helle S, Gordon A, Alfaro G, García X, Ulloa C. Coal blend combustion: link between unburn carbon in fly ashes and maceral composition. Fuel Process Technol. 2003;80:209–23.
Ulloa C, Borrego AG, Helle S, Gordon AL, García X. Char characterization and DTF assays as tools to predict burnout of coal blends in power plants. Fuel. 2005;84:247–57.
Sarkar P, Mukherjee A, Sahu SG, Choudhury A, Adak AK, Kumar M, Choudhury N, Biswas S. Evaluation of combustion characteristics in thermogravimetric analyzer and drop tube furnace for Indian coal blends. Appl Therm Eng. 2013;60:145–51.
Vamvuka D, Sfakiotakis S. Combustion behaviour of biomass fuels and their blends with lignite. Thermochim Acta. 2011;526:192–9.
Gil MV, Casal D, Pevida C, Pis JJ, Rubiera F. Thermal behaviour and kinetics of coal/biomass blends during co-combustion. Bioresour Technol. 2010;101:5601–8.
Wu T, Gong M, Lester E, Hall P. Characteristics and synergistic effects of co-firing of coal and carbonaceous wastes. Fuel. 2013;104:194–200.
Acknowledgements
The authors are thankful to Director, CSIR-Central Institute of Mining and Fuel Research for giving permission to publish the paper. Some portions of the work presented in this paper have been incorporated in the Ph. D thesis of Sri S. G. Sahu submitted to Jadavpur University, Kolkata, India.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sarkar, P., Sahu, S.G., Chakraborty, N. et al. Studies on potential utilization of rice husk char in blend with lignite for cocombustion application. J Therm Anal Calorim 115, 1573–1581 (2014). https://doi.org/10.1007/s10973-013-3499-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-013-3499-z