Abstract
Dark chocolate is a complex food product in which sugar crystals and cocoa particles are surrounded by cocoa butter. Thermogravimetry and derivative thermogravimetry are proposed as fast, cheap, and sensitive tools to determine the composition of dark chocolate and consequently to confirm the cocoa percent declared by the producer or to check the production cycle.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fast and sensitive methodologies of food analysis, especially for industrial purposes, are useful tools to determine the quality of final commercial products. Moreover, the possibility to use always easier tools to analyze the authenticity of the certificated food composition is a daily challenge. Thermal analysis is recognized as instrumental method of food analysis able to give unique informations regarding the nature of the sample or the modifications induced by industrial processing. Books and reviews report the applications of thermoanalytical techniques to the food science [1–5].
Chocolate is a complex suspension of around 70 % of fine solid particles (from sugar and cocoa), in a continuous fat phase. At ambient temperature (around 25 °C), it is solid and it melts at oral temperature (37 °C) generating a smooth suspension of solid particles in cocoa butter. There are different types of chocolate (dark, milk, and white), according to their composition in terms of cocoa solids, milk fat, and cocoa butter, hence the final products have different compositions. “Dark” chocolate is a complex food product in which sugar crystals and cocoa particles are surrounded by a continuous phase of crystalline and liquid cocoa butter. Due to the hydrophilic nature of the sugar crystals, a small portion of emulsifier, e.g., soy lecithin or polyglycerol polyricinoleate (PGPR), is often added to improve compatibility with the hydrophobic cocoa butter [6, 7]. Thermal analysis has been extensively applied to characterize the thermal, structural, and rheological properties of the dark chocolate [8–10].
The aim of this work, on the basis of our group experience in thermal analysis [11–23] and in food characterization [24, 25], was to propose the thermogravimetry (TG) and the derivative thermogravimetry (DTG) as fast, simple, cost-effective, and sensitive tools to determine the composition of dark chocolate and consequently to control the dark chocolate quality and to certify the cocoa percent declared by the producer.
Experimental
Material
All the analyzed materials were either purchased in specialized stores (brands are omitted) or kindly gifted by chocolate producing companies: five chocolate samples declared as 40 % cocoa (40 %-C chocolate), two 47 % cocoa (47 %-C chocolate), five 70 % cocoa (70 %-C chocolate), 85 % cocoa (85 %-C chocolate), 99 % cocoa (99 %-C chocolate), three different sugars (S), three different cocoa liquor (CL), and four different cocoa butter (CB).
Instrumental
The thermoanalytical curves were obtained by using a Perkin Elmer TG7 thermobalance (range 20–1,000 °C); the atmosphere was either pure nitrogen or air, at a flow rate of 100 mL min−1; the heating rate was varied between 5 and 40 °C min−1, with the best resolution achieved at a scanning rate of 10 °C min−1.
Results and discussion
Although the U.S. has no official definition for dark chocolate, European rules specify a minimum of 35 % cocoa solids [26]. In addition to very specific and sensitive analytical approaches already published [27–29], a fast, cost-effective, and simple tool to determine the percent composition of the main ingredients could be very useful to control the effectiveness of the declared quality. To this end, thermogravimetric analysis (TG) and first DTG were performed on purchased dark chocolate samples. The reproducibility of the TG experiments was always good, with a fluctuation below 2 %. At least three experiments were performed for each sample, but the number was up to five or six experiments for those samples with fluctuations higher than 0.5 %.
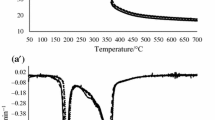
The typical thermogravimetric behavior of a dark chocolate sample is shown in Fig. 1 by a 70 % cocoa declared TG profile.
Under nitrogen purging flow, the TG curve shows three main steps in the temperature range 20–210, 210–340, and 340–750 °C, the second step being the convolution of at least three processes.
By the analysis of the starting materials (sugar, cocoa liquor, and cocoa butter, Fig. 2) it is very easy to assign each TG step in the dark chocolate curve: the first one is related to the sugar contribution, the second step is the release of the cocoa liquor and the final loss is related to the cocoa butter. Figure 3 clearly shows the correspondence.
For the starting materials the reproducibility was high, with results never different of more than 0.3 %.
The nitrogen purging flow gives a clear qualitative profile, but the quantitative interpretation of the analysis is not allowed since inert flows depress the complete decomposition, without a final constant mass value.
By changing the atmosphere to air, a final plateau is obtained, thus allowing quantitative calculations. The TG and DTG curves of Fig. 4 show that the presence of oxygen has no effect on the first release, while shifts to lower temperature, the second mass loss step mainly influences the third one.
Figure 4 clearly shows that the quantitative results have to be calculated from the curves recorded under air flow. By this method, in Table 1 the calculated and declared cocoa percent for each analyzed dark chocolate sample have been reported. The cocoa is the sum of cocoa liquor and cocoa butter. The higher fluctuation in the lower percent cocoa samples has been attributed to the higher amount of excipients that influences the shape of the curve and consequently the calculated cocoa percent. At least five TG experiments have been run for the 40 %-C samples.
It is interesting to note that there is a clear sugar/cocoa dependence when considering different chocolate amount in products of the same brand. This trend is clearly enhanced in Fig. 5, where several representative TG curves show a decrease of the sugar peak when the cocoa percent increases.
A final comment is due for samples with cocoa <70 %: in the final TG step, the derivative curve often shows an additional overlapped peak that is due to excipients (i.e., vanillin) and must be subtracted to avoid wrong evaluations. This process lowers intensity or disappears in samples with higher cocoa percent. In Table 1 all the results have been subtracted by this contribution.
Conclusions
TG and DTG revealed to be useful tools for the determination of the dark chocolate composition. The results show the possibility to give fast, cost-effective, and accurate informations when the purging flow is air and to propose TG and DTG for the control and the certification of the dark chocolate quality.
References
Kemp RB, editor. Handbook of thermal analysis and calorimetry: from macromolecules to man – Chapter 16: thermal analysis in foods and food processing. Amsterdam: Elsevier; 1999.
Gabbot P, editor. Principles and applications of thermal analysis. Oxford: Wiley; 2007.
Materazzi S, Vecchio S. Recent applications of evolved gas analysis by infrared spectroscopy (IR-EGA). Appl Spectrosc Rev. 2013;48(8):654–89.
Materazzi S, Vecchio S. Evolved gas analysis by mass spectrometry. Appl Spectrosc Rev. 2011;46(4):261–340.
Materazzi S, Vecchio S. Evolved gas analysis by infrared spectroscopy. Appl Spectrosc Rev. 2010;45:241–73.
Garti N, Wildak NR, editors. Cocoa butter and related compounds. Urbana: AOCS publishing; 2012.
Beckett ST. The science of chocolate. Cambridge: The Royal Society of Chemistry; 2000.
Fernandes VA, Müller AJ, Sandoval AJ. Thermal, structural and rheological characteristics of dark chocolate with different compositions. J Food Eng. 2013;116:97–108.
Svanberg L, Ahrné L, Lorén N, Windhab E. Impact of pre-crystallization process on structure and product properties in dark chocolate. J Food Eng. 2013;114:90–8.
Glicerina V, Balestra F, Dalla Rosa M, Romani S. Rheological, textural and calorimetric modifications of dark chocolate during process. J Food Eng. 2013;119:173–9.
Vecchio S, Cerretani L, Bendini A, Chiavaro E. Thermal decomposition study of monovarietal extra virgin olive oil by simultaneous TG/DSC: relation with chemical composition. J Agric Food Chem. 2009;57:4793–800.
Campanella L, Nuccilli A, Tomassetti M, Vecchio S. Biosensor analysis for the kinetic study of polyphenol deterioration during the forced thermal oxidation of extra virgin olive oil. Talanta. 2008;74:1287–98.
Vecchio S, Campanella L, Nuccilli A, Tomassetti M. Kinetic study of thermal breakdown of triglycerides contained in extra-virgin olive oil. J Therm Anal Calorim. 2008;91:51–6.
Vecchio S, Materazzi S, Wo LW, De Angelis Curtis S. Thermoanalytical study of imidazole-substituted coordination compounds: Cu(II)- and Zn(II)-complexes of bis(1-methylimidazol-2-yl)ketone. Thermochim Acta. 2013;568:31–7.
Materazzi S, Vecchio S, De Angelis Curtis S. Thermal analysis and health safety. Thermoanalytical characterization of hardwood softwood dust. J Therm Anal Calorim. 2013;112:529–33.
Materazzi S, Vecchio S, Wo LW, De Angelis Curtis S. TG-MS and TG-FTIR studies of imidazole-substituted coordination compounds. Co(II) and Ni(II)-complexes of bis(1-methylimidazol-2-yl)ketone. Thermochim Acta. 2012;543:183–7.
Perrino C, Canepari S, Materazzi S. Thermal stability of inorganic and organic compounds in atmospheric particulate matter. Atmos Environ. 2012;54:36–43.
Materazzi S, Vecchio S, Wo LW, De Angelis Curtis S. Thermoanalytical studies of imidazole-substituted coordination compounds. Mn(II)-complexes of bis(1-methylimidazol-2-yl)ketone. J Therm Anal Calorim. 2011;103:59–64.
Materazzi S, Gentili A, Curini R. Applications of evolved gas analysis. Part 2: EGA by mass spectrometry. Talanta. 2006;69:781–94.
Migliorati V, Ballirano P, Gontrani L, Materazzi S, Ceccacci F, Caminiti R. A combined theoretical and experimental study of solid octyl and decylammonium chlorides and of their aqueous solutions. J Phys Chem B. 2013;117:7806–18.
Materazzi S, Foti C, Crea F. Nickel and Copper biomimetic complexes with N-heterocyclic dicarboxylic ligands. Thermochim Acta. 2013;573:101–105.
Bretti C, Crea F, De Stefano C, Foti C, Materazzi S, Vianelli G. Thermodynamic properties of Dopamine in aqueous solution. Acid − base properties, distribution, and activity coefficients in NaCl aqueous solutions at different ionic strengths and temperatures. J Chem Eng Data. 2013;58:2835–2847.
Serra Moreno J, Panero S, Materazzi S, Martinelli A, Sabbieti MG, Agas D, Materazzi G. Polypyrrole-polysaccharide thin films characteristics: electrosynthesis and biological properties. J Biomed Mat Res Part A. 2009;88:832–840.
Tabilio MR, Fiorini D, Marcantoni E, Materazzi S, Delfini M, De Salvador FR, Musmeci S. Impact of the Mediterranean Fruit Fly (Medfly) Ceratitis Capitata on different peach cultivars: the possible role of peach volatile compounds. Food Chem. 2013;140:375–81.
Bevilacqua M, Bucci R, Materazzi S, Marini F. Application of near infrared (NIR) spectroscopy coupled to chemometrics for dried egg-pasta characterization and egg content quantification. Food Chem. 2013;140:726–34.
Directive 2000/36/EC of the European Parliament and of the Council of 23 June 2000 relating to cocoa and chocolate products intended for human consumption”. Eur-lex.europa.eu.
Dóka O, Prágai E, Bicanic D, Kulcsár R, Ajtony Z. Colorimetry and photoacoustic spectroscopy as a suitable tool for determination of fat-free cocoa solids in dark chocolates. Eur Food Res Technol. 2013;236:963–8.
Bignardi C, Mattarozzi M, Penna A, Sidoli S, Elviri L, Careri M, Mangia A. A rapid size-exclusion solid-phase extraction step for enhanced sensitivity in multi-allergen determination in dark chocolate and biscuits by liquid chromatography–tandem mass spectrometry. Food Anal Methods. 2013;6:144–1152.
Grassi D, Desideri G, Necozione S, Ruggieri F, Blumberg JB, Stormello M, Ferri C. Protective effects of flavanol-rich dark chocolate on endothelial function and wave reflection during acute hyperglycemia. Hypertension. 2012;60:827–32.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Materazzi, S., De Angelis Curtis, S., Ciprioti, S.V. et al. Thermogravimetric characterization of dark chocolate. J Therm Anal Calorim 116, 93–98 (2014). https://doi.org/10.1007/s10973-013-3495-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-013-3495-3