Abstract
The study of the behaviour of cellulose materials at low doses of ionizing radiation regained the interest because of the recent results showing that physical properties of the paper have less or no changes for absorbed doses below 10 kGy, despite the high decrease of the degree of polymerization. The understanding of the relationship among molecular, microscopic and macroscopic changes in cellulose materials may change the current opinion that irradiation of paper is not the best choice for conservation of cultural heritage. The aim of this study is to reveal the changes in gamma-irradiated pure cellulose paper by simultaneous TG/DSC analysis. For cellulose fibres, the thermal decomposition parameters depend on the cellulose degree of polymerization. For high irradiation doses, there is established a relationship between the absorbed dose and the degree of polymerization. However, a direct relationship between absorbed dose and the parameters of cellulose thermal decomposition for low irradiation doses was not established either in the literature or in our study. By using a peak separation technique, we studied the changes in the region of water loss (70–150 °C) and physical ageing (160–300 °C) for Whatman paper with low initial water content (<1 %), previously gamma irradiated at doses between 0 and 30 kGy. We concluded that strength of the hydrogen bond structure is increasing up to a point when the stress produces fractures in the fibrilar structure. This may explain the results reported for mechanical tests at low dose irradiation and it is in agreement with scanning electron microscopy pictures showing changes in fibril structure at high irradiation doses. Cellulose irradiated at low doses maintains its original hydrogen bond structure despite the decrease of the degree of polymerization.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Paper is the main support for writing since the development of industrial paper making machines. Because of its degradability, the huge written heritage of the last centuries is difficult to be preserved. Beside some technological errors (rosin sizing, iron gall ink), another main issue is the biological degradation. Uncontrolled humidity and temperature variations in storage conditions, caused by unpredictable events (floods and other natural disasters, wars, etc.) or simply the lack of care are always followed by a massive attack of micro-organisms. Once paper is attacked; the biological contamination is difficult to remove, and in the field of paper conservation, there is a lack of efficient mass decontamination techniques. Among others (fumigation, oxygen deprivation), irradiation with high-energy ionizing radiation seems to be the most effective because of the certainty of the microbicidal effect [1–3].
Irradiation treatment showed no destructive effects on some materials from cultural heritage like wood, parchment, or stone paintings [4–6], but in the case of paper, it was obvious from the beginning a major decrease of cellulose degree of polymerization [7, 8]. For that reason, ionizing radiation is not considered the best choice for the conservation of paper [9, 10]. Being the most widespread biopolymer, the radiolysis of cellulose was on high interest, and a relationship between the absorbed dose and number of scissions or degree of polymerization was early established [11]. Supplementary data were added in studies connected to irradiation of mail as a measure against bio-terrorism threats [12, 13]. Important decrease of DP in low-dose irradiation range was confirmed for Whatman (pure cellulose) paper [14] and other sorts of commercial paper [15, 16].
Despite the intensive decrease of the degree of polymerization, other physical properties of the paper have less or no changes for irradiation at doses below 10 kGy [14–19]. There were reported no significant differences for mechanical test results (tensile strength, elongation at break) for doses up to 5 kGy for paper samples irradiated in different conditions (in nitrogen atmosphere, in vacuum or soaked with water) and exposed to accelerated ageing [14, 15]. Experimental data show sometime an improvement of these properties, but it was considered the effect of the high uncertainty of the measurement of the mechanical testing methods [14–16, 19, 20]. This type of behaviour is usually explained by crosslinking, but this is rarely reported for pure cellulose [12, 21, 23]. Because the direct determination of crosslinks in the irradiated cellulose is impossible, indirect methods like study of physical–chemical processes that are associated with the mobility of macro-chains should be applied [23]. In the absence of new functional groups within the polymer moiety, the observed crosslinking effects of irradiated cellulose may be assigned to an increase in the hydrogen bond supramolecular structure.
For paper, it is well known the importance of the hydrogen bonds, the basics of supramolecular cellulose structure and the three-dimensional network of fibres [21]. Hydrogen bonds are considered to be responsible for the stability of cellulose compositions over a large range of temperatures [22]. In paper industry, it is known the effect of formation and loss of cellulose intermolecular hydrogen bonds when water is removed or added to paper. It is known the hysteresis of the mechanical properties (5–25 %) when humidity of paper is changed, and Technical Association of the Pulp and Paper Industry (TAPPI) recommends the preconditioning of the samples at 20 % R.H. for erasing the previous temperature/humidity history of the paper samples [24]. The glass transition of amorphous cellulose was studied by differential scanning calorimetry [25–29], dynamical mechanical analysis [30] and computer simulation [31] and it was reported a shift of the glass transition temperature from temperatures below 100 °C to temperatures over 200 °C when the water content of the samples decreases to 0.
Thermal analysis proved its potential for characterization of cellulose modification processes in studies related to wood, fabrics or paper ageing [25, 32–36], to fibres used in fabrics [37] and composites [38] or to biomass production [39–41]. Experimental results show a direct relationship between the degree of polymerization of cellulose and certain thermal degradation parameters [42].
The aim of this study is the investigation of the changes in gamma-irradiated pure cellulose paper by simultaneous TG/DSC analysis and to find the correlation between peak parameters and the changes in cellulose structure.
Materials and methods
The tests were performed on Whatman 1 CHR paper (pure cellulose-chromatographic grade) produced from cotton linters with no additives [43]. Samples where cut in discs of about 5.5 mm (close to the size of the Al crucibles) with a standard perforating machine. Netzsch STA 409 PC Luxx Simultaneous Thermal Analyzer with TG/DSC sample carrier was used for testing under inert atmosphere (50 mL min−1 pure nitrogen). A quantity of about 16 mg was tested between 40 and 600 °C at 10 K min−1 in aluminium crucibles with pierced caps. For reducing the influence of free water, the samples were conditioned at least 24 h at 5 % R.H. at room temperature. Each measurement was performed in triplicate. For analysis of the DSC curves, it was used a peak separation technique [44].
Samples were irradiated at the following doses: 2.9 ± 0.2, 5.7 ± 0.5, 8.4 ± 0.8, 14.1 ± 1.2, 28.2 ± 2.2 kGy, (confidence interval given with 2σ), in the SVST Co-60 gamma irradiator of IRASM Radiation Processing Center (IFIN-HH) at 9.3 kGy h−1. The irradiator is a tote-box industrial type, but experimental arrangements allow static irradiation. The dose range was chosen to overlap the dose range of interest for the irradiation of cultural heritage items [14–17, 45]. The absorbed dose was measured with ethanol–chlorobenzene by oscillometric method.
Results and discussion
Typical TG/DTG/DSC curves [46] obtained in our experiment are shown in Fig. 1. For noise reduction in the representation of DTG curves a smooth factor = 5, it was applied to the original TG curve. TG and DSC curves are represented with original data.
Four distinctive regions can be observed on the TG/DTG/DSC curves: I—the region of water loss (around 100 °C); II—the region with practically no mass loss (around 250 °C); III—the region of cellulose thermal decomposition (around 365 °C); IV—the region of char processes (over 400 °C).
Considering the low sensitivity of the DSC over 400 °C and the random appearance of artefacts which may be assigned to char phase transitions, the region of over 400 °C was not taken into consideration in our analysis.
The water loss (I) is obvious in DSC curves but almost hidden in TG/DTG curves (less than 1 % water loss). Because of the reduced ratio of water (water evaporating/desorbing below 100 °C), only one peak is observed on DSC curves for 10 K min−1 heating rate [47]. The peak temperature over 100 °C is showing the main contribution of bond water [27, 48, 49].
Region (II) shows a similar DSC profile for all the absorbed doses and practically no mass loss. This profile can be assigned to cellulose de-polymerization (ageing) [29, 30] and glass transition [25, 27, 29] combined effect. Such effect is usually observed around 60 °C [29], but it was reported also around 250 °C [46], as in our results. This shift to higher temperature may be assigned to the strengthening of the hydrogen bond structure in the samples with low water content. An increased number of intra- and inter-molecular hydrogen bonds will preserve the reduced macromolecule mobility for a larger temperature range. There is no obvious glass transition in that area in the DSC curves and this may be explained by the random intermolecular hydrogen bonding of the amorphous cellulose. The paper structure is inhomogeneous at microscopic level so the phase transitions should be considered within the boundary of each structural unit (fibres, fibrils, micro-fibrils).
Taking into account the shape of the obtained DSC curves, we chosen to describe the region (II) with an asymmetrical gaussian peak in the further described peak separation. The shape and position of this peak should depend on both the initial degree of polymerization and strength of the hydrogen bond structure.
Third region (III) shows the usual profile of thermal decomposition of cellulose [46]. As expected for Whatman paper, there is a high peak temperature, related to the high molecular mass of cotton cellulose and there are no obvious evidences of mixture of different grade pulps (no shoulders in the DTG/DSC peaks). Position and shape of this peak should depend on the initial degree of polymerization [42, 50] which should be supplementary decreased by the de-polymerization (ageing) stage.
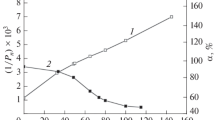
Figure 2 shows the dependence of the main thermal analysis parameters on the absorbed dose for the thermal decomposition of cellulose (region III). The error bars represent the standard deviation of three samples for each absorbed dose.
For doses higher than 10 kGy, there is a uniform decrease or increase of the decomposition parameters, but there is no obvious dose dependence below 10 kGy. The high sensitivity of the thermal analysis methods (the coefficient of variation is better than 1 % for most of the parameters shown in Fig. 2) allows this conclusion when mechanical tests can show only no statistical differences for a similar range of doses [14, 15, 19]. Thermal analysis samples are much smaller than mechanical testing samples, and their uniformity is less affected by individual flaws in paper structure.
The highest coefficient of variation was obtained for temperature onset of the DTG peaks and this is due to an artefact which is present on all the DTG curves, with no correspondence on the DSC curves, as it can be seen in the example from Fig. 3. This may be due to the contraction of the sample during the char formation: the macroscopic movement of fibres is following the microscopic movement of fibrils when the backbone molecules of char are aggregating. An interesting fact is that the temperature of this phenomena (Tb in the plot from Fig. 2a) follows well the profile of the peak temperature so the char formation should be depend mainly on this parameter.
We obtained higher residual mass (Fig. 2b) at the end of decomposition process when dose is increased which suggests a retard of the process for lower degree of polymerization.
The only parameter which shows a continuous increase (in absolute value) with the increase of absorbed dose is the maximum mass change rate. The speed of reaction is probably the most sensitive parameter to the modification of the degree of polymerization [42]. All other parameters show a non-uniform behaviour below 10 kGy. However, it should be mentioned the decrease of the peak width which is opposite to the widening of DP distribution for irradiated cellulose [12, 51].
The non-uniform behaviour of the cellulose peak decomposition parameters may not be explained by the decrease of degree of polymerization alone. The continuous decrease of the degree of polymerization was reported at both low and high irradiation doses [11–15]. The hydrogen bond supramolecular structure, which is known to be considerable in the case of cellulose paper, cannot affect directly the decomposition peak because of the high temperature range. Under these circumstances, the study should be extended to the processes before the thermal decomposition of cellulose.
By using a peak decomposition technique [44], we obtained supplementary information on the changes induced by the ionizing radiation to the hydrogen bond structure of cellulose paper.
Figure 4 shows the separated peaks for the DSC curves from Fig. 1 in a linear base line approximation. Non-linear regression (w = 1 that is non-weighted least squares) was performed for three peaks on a range close to the onset of the water loss peak and end of the cellulose decomposition peak.
Despite the first two peaks seems to be almost symmetrical (gaussian), we chosen for all three peaks a Fraser-Suzuki shape (asymmetric gaussian) [52] available in Netzsch peak separation software. For all peak separations, it was obtained a correlation coefficient better than 0.99918.
The plots in Figs. 5 and 6 show the dose dependence for the parameters of the separated peaks. There is almost no overlapping for the first two peaks, but the overlapping of the second and third peak is increasing with dose. When absorbed dose increases, there is shift of peak 2 to higher temperatures and a shift of peak 3 to lower temperatures.
There are no significant changes for the values and the shape of the dose dependence for the parameters of the cellulose decomposition peak (Peak 3) compared with the dose dependences from Fig. 2. The asymmetry of the peak (Fig. 6c) shows no significant changes for the dose range on study.
A non-uniform behaviour at low doses was obtained also for the first and second peak.
Because of the low water content and the lack of sensibility of the instrument for temperatures below 70 °C, the parameters of the water loss peak (peak 1) have a higher coefficient of variation. Both position (peak temperature) and width of the peak are changed when the dose is increased (Fig. 5b, c). For both, it can be observed a step function: over 10 kGy, there is a sudden broadening of the DSC peak which in maintained almost constant for doses higher than 10 kGy. Consequently, the peak area presents the same shape (Fig. 5d).
The standard deviations of peak 2 onset and endset are considerable higher than the standard deviation of other parameters shown in Fig. 5a. This is due to weak constraints in the peak separation that allows a weaker convergence of the non-linear regression. Peak 2 width and asymmetry are also mostly influenced by the linear baseline approximation. However, peak temperature and peak amplitude have better coefficient of variation and show a similar behaviour: almost constant value below 10 kGy and a continuous increase over 10 kGy.
The asymmetry of first peak (Fig 6a) is low and is decreasing to zero (gaussian shape), within the limits of a high standard deviation. The same trend is observed for the second peak, while the third one (cellulose decomposition) is maintaining its specific asymmetry on a large interval of absorbed doses (Fig. 6b, c).
Conclusions
The analysis of the peak parameters of the cellulose thermal decomposition does not provide enough information for explaining the week dose dependence at low doses. In this temperature range, it should not be any influence of the intermolecular hydrogen bonds, but the degree of polymerization at the beginning of thermal decomposition is altered by the previous ageing stage.
Supplementary information can be obtained by peak separation. The strong hydrogen structure can delay the de-polymerization of cellulose. By breaking the cellulose macromolecule, it is expected in a first phase a change in the hydrogen bond structure: reforming of the intra- and inter-molecular hydrogen bonds or strengthening of the existing ones with an overall increase of the hydrogen intermolecular bonds (crosslink). With a further decrease of DP (increase of absorbed dose), the strengthened hydrogen bond structure may produce breakings in the structural units of the fibre (micro-fibrils, fibrils). This can explain the step function profile observed for peak 1 half width. Changes in the fibril structure were observed by scanning electron microscopy [53, 54].
For certain thermal analysis parameters (thermal decomposition mass loss, rate of thermal degradation), it was reported in the literature, a continuous (exponential) decrease when degree of polymerization is increasing [42]. It is well known the continuous decrease of degree of polymerization when irradiation dose is increased [11–18]. In our study, we obtained a non-uniform dependence on the absorbed dose for most of the thermal decomposition parameters. However, we can identify a dose range where the changes in cellulose structure are driven mainly by the hydrogen bond structure. For this dose range, the hydrogen bond structure is maintained or improved independently of decrease of degree of polymerization (up to 50 % [14, 20]). Cellulose irradiated at low doses maintains its original hydrogen bond structure, despite the decrease of the degree of polymerization, and this explains why paper maintains its mechanical properties practically unchanged [14, 15, 19].
References
Bonetti M, Gallo F, Magaudda G, Marconi C, Montanari M. Essais sur l’utilization des rayons gamma pour la sterilization des materiaux libraires. Stud Conserv. 1979;24:59–68.
Tomazello MGC, Wiendl FM. The applicability of gamma radiation to the control of fungi in naturally contaminated paper. Restaurator. 1995;16:93–9.
Tiano P. Biodegradation of cultural heritage: Decay mechanisms and control methods. In: 9th ARIADNE Workshop “Historic Material and their Diagnostic”, ARCCHIP, Prague, 22–28 April 2002. http://www.arcchip.cz/w09/w09_tiano.pdf. Accessed 12 April 2013.
Ponta CC. Irradiation conservation of cultural heritage. Nucl Phys News. 2008;18:22–4.
Nunes I, Mesquita N, Cabo Verde S, Trigo MJ, Ferreira A, Carolino MM, Portugal A, Botello ML. Gamma radiation effects on physical properties of parchment documents: assessment of Dmax. Radiat Phys Chem. 2012;81:1943–6.
Abdel-Haliema MEF, Ali MF, Ghaly MF, Sakr AA. Efficiency of antibiotics and gamma irradiation in eliminating Streptomyces strains isolated from paintings of ancient Egyptian tombs. J Cult Herit. 2013;14:45–50.
Calvini P, Santucci L. Alcuni dati sugli effetti dell’ irradiazione gamma sulla carta. Boll Inst Centr Patolog Libro. 1978;35:55–62.
Butterfield FJ. The potential long-term effects of gamma irradiation of paper. Stud Conserv. 1987;32:181–91.
Nittérus M. Fungi in archives and libraries. Restaurator. 2000;21:25–40.
Silverman R, Bliss M, Erickson H, Fidopiastis N, Francl J, Knight B, Lively K, Neuvirt J, Novotny D, Yeager N. In: Comparing mass drying and sterilization protocols for water-damaged books. National Center for Preservation Technology and Training, Materials Research Series, https://ncptt.nps.gov/blog/cpmparing-mass-dryng-and-sterilization-protocols-for-water=damaged-books-2008-04. Accessed 12 April 2013.
Charlesby A. The degradation of cellulose by ionizing radiation. J Polym Sci. 1955;15:263–70.
Bouchard J, Methot M, Jordan B. The effects of ionizing radiation on the cellulose of woodfree paper. Cellulose. 2006;13:601–10.
Zinovev OA, Skobkin VS, Lobanov NS, Chugunov OK, Pizhov GYa, Naidenov AYa, Dubinina TP. Radiation sterilization of mail. Atomic Energy. 2006;100:67–71.
Adamo M, Giovannotti M, Magaudda G, Plossi Zappalà M, Rocchetti F, Rossi G. Effect of gamma rays on pure cellulose paper as a model for the study of treatment of biological recovery of biodeteriorated books. Restaurator. 1998;19:41–59.
Adamo M, Brizzi M, Magaudda G, Martinelli G, Plossi-Zappalà M, Rocchetti F, Savagnone F. Gamma radiation treatment of paper in different environmental conditions: chemical, physical and microbiological analysis. Restaurator. 2001;22:107–31.
Area MC, Calvo AM, Felissia FE, Docters A, Miranda M, Raverta V. Influencia de la dosis de radiación y la tasa de dosis sobre las propiedades físicas de papeles comerciales usados en Bibliotecas y Archivos. Proceedings of the 45 Congresso Internacional de Celulose e Papel da ABTCP/VII Congresso Ibero-Americano de Pesquisa de Celulose e Papel; 2012. October, 9–11, 2012, Sao Paulo Brazil.
D’Almeida MLO, Barbosa PDSM, Boaratti MFG, Borrely SI. Radiation effects on the integrity of paper. Radiat Phys Chem. 2009;78:489–92.
Magaudda G. The recovery of bio-deteriorated books and archive documents through gamma radiation—some considerations on the results achieved. J Cult Herit. 2004;5:113–8.
Moise IV, Virgolici M, Negut CD, Manea M, Alexandru M, Trandafir L, Zorila FL, Talasman CM, Manea D, Nisipeanu S, Haiducu M, Balan Z. Establishing the irradiation dose for paper decontamination. Radiat Phys Chem. 2012;81:1045–50.
Talasman CM, Radu A, Manea D, Burlacu M. Physico-mechanical tests on the materials from the treated archives. http://irasm.ro/arcon/pub/3/III%20ARCON%20CEPROHART2.pdf. Accesed 12 April 2013.
Klemm D, Philipp B, Heinze T, Heinze U, Wagenknecht W. Comprehensive cellulose chemistry. Volume l: fundamentals and analytical methods. Weinheim: Wiley; 1998.
Shen T, Gnanakaran S. The stability of cellulose: a statistical perspective from a coarse-grained model of hydrogen-bond networks. Biophys J. 2009;96:3032–40.
Kovalev GV, Bugaenko LT. On the crosslinking of cellulose under exposure to radiation. High Energy Chem. 2003;37:209–15.
TAPPI Standard conditioning and testing atmospheres for paper, board, pulp handsheets, and related products. Test Method T 402 sp-08. TAPPI, 1993.
Carr DJ, Odlyha M, Cohen N, Phenix A, Hibberd RD. Thermal analysis of new, artificially aged and archival linen. J Therm Anal Calorim. 2003;73:97–104.
Batzer H, Kreibich UT. Influence of water on thermal transitions in natural polymers and synthetic polyamides. Polym Bull. 1981;5:585–90.
Szcześniak L, Rachocki A, Tritt-Goc J. Glass transition temperature and thermal decomposition of cellulose powder. Cellulose. 2008;15:445–51.
Paes SS, Sun S, MacNaughtan W, Ibbett R, Ganster J, Foster TJ, Mitchell TR. The glass transition and crystallization of ball milled cellulose. Cellulose. 2010;17:693–709.
Roig F, Dantras R, Dandurand J, Lacabanne C. Influence of hydrogen bonds on glass transition and dielectric relaxations of cellulose. J Phys D Appl Phys. 2011;44(4):045403.
Hutchinson JM. Determination of the glass transition temperature. Methods correlation and structural heterogeneity. J Therm Anal Calorim. 2009;98:579–89.
Chen W, Lickfield GC, Yang CQ. Molecular modelling of cellulose in amorphous state. Part I: model building and plastic deformation study. Polymer. 2004;45:1063–71.
Franceschi E, Palazzi D, Pedemonte E. Thermoanalytical contribution to the study on paper degradation. Characterisation of oxidised paper. J Therm Anal Calorim. 2001;66:349–58.
d’Almeida ALFS, Barreto DW, Calado V, d’Almeida JRM. Thermal analysis of less common lignocellulose fibers. J Therm Anal Calorim. 2008;91:405–8.
Budrugeac P, Emandi A. The use of thermal analysis methods for conservation state determination of historical and/or cultural objects manufactured from lime tree wood. J Therm Anal Calorim. 2001;104:707–16.
Melniciuc-Puica N, Lisa G, Rusu I. On the lifetime prediction of old documents. J Therm Anal Calorim. 2010;99:835–42.
Zervos S, Moropoulou A. Methodology and criteria for the evaluationof paper conservation interventions. A literature review. Restaurator. 2007;27:219–74.
Corradini E, Teixeira EM, Paladin PD, Agnelli JA, Silva ORRF, Mattoso LHC. Thermal stability and degradation kinetic study of white and colored cotton fibers by thermogravimetric analysis. J Therm Anal Calorim. 2009;97:415–9.
Yao F, Wu Q, Zhou D. Thermal decomposition of natural fibers: global kinetic modeling with nonisothermal thermogravimetric analysis. J Appl Polym Sci. 2009;114:834–42.
Kaloustian J, Pauli AM, Pastor J. Kinetic study of the thermal decompositions of biopolymers extracted from various plants. J Therm Anal Calorim. 2001;63:7–20.
Yang H, Yan R, Chen H, Lee DH, Zheng C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel. 2007;86:1781–8.
Korosec RC, Lavric B, Rep G, Pohleven F, Bukovec P. Thermogravimetry as a possible tool for determining modification degree of thermally treated Norway spruce wood. J Therm Anal Calorim. 2009;98:189–95.
Sperova M, Nasadil P, Prusova A, Kucerık J. A hint on the correlation between cellulose fibers polymerizationdegree and their thermal and thermo-oxidative degradation. J Therm Anal Calorim. 2012;110:71–6.
Whatman 1 CHR Chromatography Paper. http://www.fishersci.com/ecomm/servlet/fsproductdetail_10652_5529506_29104_-1_0. Accesed 12 April 2013.
Netzsch Peak separation version 2006.01, Netzsch Geraetebau Gmbh.
Gonzalez ME, Calvo AM, Kairiyama E. Gamma radiation for preservation of biologically damaged paper. Radiat Phys Chem. 2002;63:263–5.
Soares S, Caminot G, Levchik S. Comparative study of the thermal decomposition of pure cellulose and pulp paper. Polym Degrad Stab. 1995;49:275–83.
Prusova A, Smejkalova D, Chytil M, Velebny V, Kucerik J. An alternative DSC approach to study hydration of hyaluronan. Carbohydr Polym. 2010;82:498–503.
Hatakeyama T, Nakamura K, Hatakeyama H. Vaporization of bound water associated with cellulose fibres. Thermochim Acta. 2000;352–353:233–9.
Princi E, Vicini S, Pedemonte E, Arrighi V, McEwen I. Thermal characterisation of cellulose based materials. Investigation of water content. J Therm Anal Calorim. 2005;80:369–73.
Bratu E, Moise IV, Cutrubinis M, Negut DC, Virgolici M. Archives decontamination by gamma irradiation. Nukleonika. 2009;54:77–84.
Sjöholm E, Gustafsson K, Norman E. Fibre strength in relation to molecular weight distribution of hardwood kraft pulp. Degradation by gamma irradiation, oxygen/alkali or alkali. Nord Pulp Paper Res. 2000;15:326–32.
Netzsch. Peak separation version. http://www.therm-soft.com/english/peaksep.htm. Accessed 12 April 2013.
Borsa J, Toth T, Takacs E, Hargittai P. Radiation modification of swollen and chemically modified cellulose. Radiat Phys Chem. 2003;67:509–12.
Han SO, Choi HY. Morphology and surface properties of natural fiber treated with electron beam. In: Méndez-Vilas A., Díaz J, editors. Microscopy: science, technology, applications and education. Formatex Research Center. 2010. pp. 1880–87. http://www.formatex.org/microscopy4/. Accesed 12 April 2013.
Acknowledgements
This study was supported by the Romanian National Authority for Scientific Research, Executive Unit for Financing Higher Education, Research, Development and Innovation (UEFISCDI), project TEXLECONS, Contr. No. 213/2012 and project ETCOG, Contr. C3-05 IFA-CEA/2012.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moise, I.V., Stanculescu, I. & Meltzer, V. Thermogravimetric and calorimetric study of cellulose paper at low doses of gamma irradiation. J Therm Anal Calorim 115, 1417–1425 (2014). https://doi.org/10.1007/s10973-013-3476-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-013-3476-6