Abstract
Differential scanning calorimetry (DSC) has become a popular tool to investigate thermal transitions in food ingredients such as gelatin. Upon heating commercial gelatin samples beyond glass transition (T g) and melting (T m) temperatures, a relatively large endothermic transition (T i) can be observed. We have observed that both the peak temperature and the enthalpy of the T i transition are influenced by the integrity of the seal of the DSC pans used for the analysis. This study shows that escape of moisture from the DSC pan appears to be responsible for this effect. The effect of different types of DSC pans, as well as technique of sealing them on the T i transition were evaluated using DSC, SDT, and TG–MS.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Gelatin is a non-homogeneous but chemically well defined, translucent, odorless, nearly tasteless solid material obtained upon extraction and hydrolysis of the collagen from animal bones, connective tissues or skin [1]. Aqueous solutions of gelatin are in the sol state at 40 °C and form thermo-reversible gels upon cooling due to the recovery of collagen triple helices via a conformational disorder–order transition of the chains [2]. This behavior lends unique organoleptic properties to gelatin which has led to its use as a gelling, thickening, and stabilizing agent in foods [3]. Being a fibrous protein, gelatin is effectively plasticized by water and alcohols, which results in lowering of glass transition temperature (T g) and unfolding (T m) [4, 5]. This has serious impact on the processing, storage, and texture of gelatin-containing foods such as ice creams, marshmallows, Jell-O™, etc. Understanding the thermal properties of gelatin is therefore vital to food technologists.
Differential scanning calorimetry (DSC) has been used as a prominent tool to investigate the thermal properties of gelatin in various forms [4–12]. DSC involves subjecting a sample and a reference pan to the same temperature profile and measuring heat flow. It is a technique, which can accurately determine the temperatures and enthalpies associated with phase transitions and has therefore found widespread application in the food industry [13]. The technique relies on the sample and reference pans being identical, both in construction and in contact with the heat source [14]. For relatively high moisture materials, such as food systems or liquids, results can be affected by the escape of volatiles from the DSC pan [15, 16].
As known in the literature, a DSC heating scan of commercial gelatin (containing ~10 mass% moisture) reveals a second order glass transition (T g) in the 80–90 °C range, followed by a first order endothermic transition (T m) in the 110–115 °C range [17, 18]. According to Slade and Levine [18] the T g is attributed to the mobility of the amorphous fringes, while T m is a result of the melting of the crystalline junction zones.
In 1989, Slade et al. [19] reported a large first-order endothermic transition above the T g and T m in the DSC scans of high bloom commercial pigskin (295 bloom) and calfskin (275 bloom) gelatin samples containing ~8–10 % moisture. They explained this newly discovered transition with peak temperature at ~170 °C, having an enthalpy (normalized per unit mass) of 20–50 cal g−1 (~85–210 J g−1), on the basis of isomerization of the peptide bonds that constitute the polyproline-II helixes of gelatin from the low energy trans to the high energy cis configuration and termed the peak temperature as T i. Such a change in structure would account for the relatively large energy input and also the reported loss in gel-forming ability (bloom strength) of the material after it underwent this thermal transition. They also found that T i increased with decreasing moisture content (until 35 % moisture, after which it remained constant) [19]. However, in 2005 D’Cruz and Bell [4] observed by inspection of DSC pans (heated to several temperatures within the T i transition), that this large endotherm was associated with irreversible sample discoloration (from yellowing at onset to browning at the end of the peak). Further, Apostolov et al. [5] reported the gradual release of water upon heating native and crosslinked gelatin samples of low moisture content (0–37 % moisture) at 10 °C min−1 from 25 to 300 °C on a thermogravimetric analyzer (TG).
Recently, Rahman et al. published work on the state diagram of bovine gelatin wherein the endothermic T m peak (according to the Slade et al. nomenclature [18, 19]) was identified as the temperature of thermal unfolding of protein and the T i peak was described as the temperature of solids-melting [20]. They also reported that the peak temperature of the higher temperature endothermic transition increased with decreasing moisture content. The same authors also reported differences between the temperature and enthalpy values of this transition for commercial bovine and porcine gelatin (with ~10 % moisture) and that of gelatin from shaari fish skin [21].
The objective of this work was to better understand the phenomena associated with this large endothermic peak, T i that follows T m (according to the Slade et al. nomenclature [18, 19]). In this study, the effect of using different types of DSC sample pans and sealing techniques on the peak temperature and enthalpy of the T i transition was investigated using standard DSC, as well as techniques such as simultaneous DSC–TG (SDT) and TG–mass spectrometry (TG–MS). SDT is a combined technique wherein DSC and TG data are collected simultaneously from the same sample, providing the advantage of identical calibration, heating conditions, and gas flow control for both signals [22]. Typically, samples are heated in an open pan and transitions involving mass loss give a concurrent endothermic signal on DSC and mass loss on TG [23]. TG–MS is a coupled evolved gas analysis (EGA) technique where the gas stream at the outlet of a TG instrument is connected to a mass spectrometer. It allows for the identification of volatile products of thermal processes based on their mass-to-charge ratios [24]. The excellent review by Giron [25] demonstrated the utility of this technique in identifying the portion of the TG mass loss curve associated with loss of water.
Experimental
Pigskin gelatin (300 bloom, Type IV) and calfskin gelatin (240 bloom, Type B) were obtained from Kraft Foods Global, Inc., Atlantic Gelatin Plant (Woburn, MA) and were tested without further preparation.
DSC experiments were performed by heating the sample from −50 to 250 °C on a TA Instruments 2920 instrument (TA Instruments, New Castle, DE). For each type of DSC pan or sealing technique employed (Table 1), an identical empty reference pan was used.
Simultaneous DSC–TG (SDT) experiments on calfskin samples were carried out by TA Instruments using a Q600SDT instrument. TG–MS was run on a TA Q500 thermogravimetric analyzer (TG) instrument (TA Instruments, New Castle, DE) connected to a Thermostar benchtop quadrupole mass spectrometer (Pfeiffer Vacuum Nashua, NH). Both these tests were run from ambient to 250 °C.
All thermal analysis experiments were carried out at heating rate of 5 K min−1 with air as the purge gas.
Encapsulation of gelatin samples in DSC sample pans
Aluminum Hermetic pans (TA instruments part# 900793.901) and lids (part# 900794.901) were used for sample encapsulation. To prepare “sealed” hermetic pans, 5–12 mg of gelatin was placed in the pan, which was then placed on a standard DSC press equipped with a hermetic sealing die (part# 900720.000). Next, the lid was placed in position and crimped using the performing tool (part# 900719.001). Once the lid was crimped in place, the pan was sealed off using the press. This last step was skipped in preparation of the “crimped only” hermetic pans. For the “pin-hole hermetic” pans, a pin hole was poked into the lid using a safety pin, prior to crimping and sealing the pans.
Stainless steel high volume pans with o-ring seals (part# 900825.902) were used for the “high volume” pan samples. In this case, 40–50 mg of sample was entered into these pans. Pans were sealed in accordance with instructions in the operator’s guide for these pans to insure appropriate sealing and safety [26]. Sample masses for all DSC pans have been provided in Table 1.
Identical reference pans were prepared for every type of sample pan used.
Results and discussion
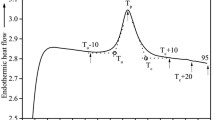
For the sake of consistency, the three thermal transitions of gelatin observed in increasing order of temperature (Fig. 1) will be referred to as T g (glass transition) < T m (melting) < T i (isomerization) in accordance to the Slade et al. [18, 19] nomenclature for the rest of this article.
DSC and SDT studies on calfskin gelatin
Two identical calfskin gelatin sample pans for every pan-type were prepared for running the separate DSC and SDT experiments. The “crimped only” hermetic pans were not run on SDT. Figure 1 shows the DSC scans of calfskin gelatin analyzed using four different types of DSC pans. Figure 2 shows the SDT scans (DSC heat flow per unit mass on Y-1 axis, TG wt. loss % on Y-2 axis and DTG, i.e., first derivative of TG wt loss on Y-3 axis). The temperature and enthalpy values of the different transitions observed from these figures are tabulated in Table 1. The four different types of DSC pans employed were expected to provide varying quality of seal integrity in the order of (best or least leaking to worst): high volume > hermetic (sealed) > hermetic (crimped only) > pin-hole hermetic.
Table 1 and Fig. 1 show that for the sample run in the pin-hole hermetic pan, which was an open system, T g and T m values could not be observed. However, samples run in the hermetic pans (both sealed and crimped only), as well as that in the high volume pan showed a T g in the 77–80 °C range and a melting peak (T m) in the 102–104 °C range. A melting enthalpy (normalized per unit mass) of 9.5–10.9 J g−1 was also observed for these samples. The values of these transitions appeared consistent with literature [4, 19]. The pin-hole hermetic pan, unlike the other pans, was a completely open system and allowed volatiles to escape from the sample during the heating scan. This can be verified from the loss of mass, which can be tracked by the decrease in the mass% signal on the Y-2 axis of Fig. 2. The sample in the pin-hole pan began to lose mass at ~70 °C and rate of mass loss continued to increase with a maximum occurring at 126.7 °C. In contrast, the hermetic (sealed) and high volume pans had lost no mass at this temperature. This continuous mass loss from the pin-hole pan caused the DSC data to be less sensitive to phase transitions occurring above 70 °C and a single large, broad endotherm, with a peak at 130.1 °C arose corresponding to the mass loss.
Upon comparing the DSC scans from the two types of hermetic pans and the high volume pan (Fig. 1), it is clear that both hermetic pans demonstrated a large endothermic T i transition while the high volume pan demonstrated a relatively small one. For the sealed hermetic pan, the enthalpy of this transition (normalized per unit mass) was 186.5 J g−1, while it was 210.2 J g−1 for the crimped only hermetic pan (Table 1). These enthalpies were within the range reported by Slade and Levine [18] for the T i transition but the enthalpy of the relatively small endotherm from the high volume pan was not. Interestingly, the endothermic peak temperatures for these transitions on both DSC (Fig. 1) and SDT (Fig. 2 Y-1 axis) appeared to increase with improving seal integrity. Upon correlating the heat flow per unit mass (Y-1), mass% (Y-2), and DTG (Y-3) axes of the SDT scan (Fig. 2), it is apparent that the large endothermic transition in the sealed hermetic pan, with a peak at 213.5 °C, corresponded to a mass loss of 12.4 %, with DTG peak at 211.8 °C. Therefore, it can be inferred that the seal of the hermetic pan failed above 200 °C, allowing escape of volatiles, which led to the appearance of a large endotherm on the DSC scans. The high volume pan, with rubber o-ring seal allowed only 0.6 % mass loss even above 200 °C and therefore the endothermic transition observed was relatively insignificant.
From Figs. 1, 2 and Table 1, it is clear that the loss of seal integrity of DSC pans allowed escape of volatiles, which corresponded to a large endotherm (T i) on the DSC scan. Also, this escape of volatiles could be prevented by performing the test in high volume DSC pans with superior seal integrity, which greatly reduced the corresponding T i endotherm. However, the chemistry of the volatile escaping from the DSC pans could not be discerned from DSC and SDT alone. To investigate this DSC and TG–MS analyses were performed. However, pigskin gelatin was used for these studies.
DSC and TG–MS studies on pigskin gelatin
Two identical pigskin gelatin sample pans for every pan-type were prepared for running separate DSC and TG–MS experiments. Figures 3 and 4 are the overlay plots of DSC and TG–MS data for pin-hole hermetic and “crimped only” hermetic pans, respectively, while Fig. 5 shows the DSC and TG scan of high volume pans. MS data were not collected for the sample in high volume pan. Since DSC and TG–MS data were not collected from the same pan, the difference in seal integrity of two identically prepared pans of the same pan-type is reflected in the data.
Overlay of DSC and TG–MS scans of pigskin gelatin in pin-hole hermetic pans. Y-1 DSC heat flow normalized to unit mass of sample (white four pointed star). Y-2 TG mass% (solid line). Y-3 MS ion current of H2O (18) and CO2 (44) (dashed line). Y-4 DTG (first derivative of mass change from TG, black four pointed star)
Overlay of DSC and TG–MS scans of pigskin gelatin in “crimped only” hermetic pans. Y-1 DSC heat flow normalized to unit mass of sample (white four pointed star). Y-2 TG mass% (solid line). Y-3 MS ion current of H2O (18) and CO2 (44) (dashed line). Y-4 DTG (first derivative of mass change from TG, black four pointed star)
Figure 3 shows a large and broad DSC endotherm for the sample in the pin-hole pan, with a peak at 83.3 °C that corresponded with a mass loss peak at 90.3 °C. The m/e 18 signal on the MS scan revealed that release of moisture through the pin-hole was detected as soon as temperature increased beyond ambient. The total water loss from the sample was 11.1 %, at the end of the DTG peak. This was the expected moisture content of the sample. Interestingly, CO2 levels (tracked on m/e 44 signal) did not increase until above 200 °C, implying that release of organic volatiles did not occur below 200 °C.
For the “crimped only” pans in Fig. 4, escape of moisture began at ~70 °C and peaked at 128 °C. The large DSC endotherm was observed at 143.2 °C. The temperature difference between the TG and DSC peaks may be attributed to the fact that the two experiments were run in separate pans though they were identically prepared. This sample released 11.15 % moisture before degradation of the hydrocarbon chains (observed by increase in CO2 levels) began above 200 °C.
The high volume pan in Fig. 5 shows a very small mass loss of 0.77 % that began after 175 °C. The DTG plot was effectively linear with no mass loss peak. Correspondingly, the DSC plot only revealed the T g and T m and a very small, broad endothermic peak (<10 J g−1) after 175 °C.
Conclusions
DSC and SDT experiments on calfskin gelatin, as well as DSC and TG–MS experiments on pigskin gelatin revealed that the T i endotherm of both commercial gelatin samples corresponded to release of moisture from the DSC pan. This endotherm has been attributed to isomerization of the polyproline [19] and more recently to solids melting [20, 21]. Our study showed that the integrity of the seal of the DSC pan affected the peak temperature and magnitude (enthalpy) of this large endotherm. The T i transition was very broad and overlapped the T g and T m if the analysis was performed in an open system, such as a pin-hole hermetic pan, which allowed easy escape of moisture. This study also demonstrated that the T i transition could be nearly eliminated (enthalpy reduced to <10 J g−1) if stainless steel high volume pan with rubber o-ring gasket was used for the analysis. This loss of water also explains the varied levels of sample discoloration observed by D’Cruz and Bell [4] upon scanning to various temperatures within the peak and opening the DSC pans thereafter. Thus, the loss of bloom strength of gelatin when processed above the T m may be associated with the loss of water.
This study underlines the role that loss of volatiles can play on DSC data and hence, importance of selecting the proper type of DSC pans for analyzing high moisture samples like food materials. More importantly, it demonstrates the value of correlating DSC with TG data and also the advantages of using combined techniques such as SDT or coupled techniques such as TG–MS to enhance data interpretation.
References
Eastoe JE. Chemical constitution of gelatin. In: Ward AG, Courtis A, editors. The science and technology of gelatin. London: Academic; 1977. p. 73–82.
Michon C, Cuvelier G, Relkin P, Launay B. Influence of thermal history on the stability of gelatin gels. Int J Biol Macromol. 1997;20:259–64.
Baziwane D, He Q. Gelatin: the paramount food additive. Food Rev Int. 2003;19:423–35.
D’Cruz NM, Bell LN. Thermal unfolding of gelatin in solids as affected by the glass transition. J Food Sci. 2005;70:E64–8.
Apostolov AA, Fakirov S, Vassileva E, Patil RD, Mark JE. DSC and TGA studies of the behavior of water in native and crosslinked gelatin. J Appl Polym Sci. 1999;71:465–70.
Tseretely GI, Smirnova OI. DSC study of melting and glass transition in gelatins. J Therm Anal Calorim. 1992;38:1189–201.
Nazzal S, Wang Y. Characterization of soft gelatin capsules by thermal analysis. Int J Pharm. 2001;230:35–45.
Fakirov S, Cagiao ME, Calleja FJB, Sapundjieva D, Vassileva E. Melting of gelatin crystals below glass transition temperature: a direct crystal-glass transition as revealed by microhardness. Int J Polym Mater. 1999;43:195–206.
Cuppo F, Venuti M, Cesàro A. Kinetics of gelatin transitions with phase separation: T-jump and step-wise DSC study. Int J Biol Macromol. 2001;28:331–41.
Sobral PJA, Habitante AMQB. Phase transitions of pigskin gelatin. Food Hydrocoll. 2001;15:377–82.
Rahman MS, Al-Saidi GS, Guizani N. Thermal characterisation of gelatin extracted from yellowfin tuna skin and commercial mammalian gelatin. Food Chem. 2008;108:472–81.
Dai C-A, Chen Y-F, Liu M-W. Thermal properties measurements of renatured gelatin using conventional and temperature modulated differential scanning calorimetry. J Appl Polym Sci. 2006;99:1795–801.
Farkas J, Mohácsi-Farkas C. Application of differential scanning calorimetry in food research and food quality assurance. J Therm Anal Calorim. 1996;47:1787–803.
Crighton JS, Wilburn FW. The role of heat transfer in the production of DSC curves. Thermochim Acta. 1992;203:1–5.
Yu L, Christie G. Measurement of starch thermal transitions using differential scanning calorimetry. Carbohydr Polym. 2001;46:179–84.
MacNeil DD, Trussler S, Fortier H, Dahn JR. A novel hermetic differential scanning calorimeter (DSC) sample crucible. Thermochim Acta. 2002;386:153–60.
Marshall AS, Petrie SE. Thermal transitions in gelatin and aqueous gelatin solutions. J Photogr Sci. 1980;28:128–34.
Slade L, Levine H. Polymer-chemical properties of gelatin in foods. In: Pearson AM, Dutson TR, Bailey AJ, editors. Advances in meat research: collagen as a food. vol. 4, New York: AVI; 1987, pp. 251–66.
Slade L, Levine H, Finley JW. Protein-water interactions: water as a plasticizer of gluten and other polymers. In: Phillips RD, Finley JW, editors. Protein quality and the effects of processing. New York: Marcel Dekker, Inc.; 1989. p. 9–124.
Rahman MS, Al-Saidi G, Guizani N, Abdullah A. Development of state diagram of bovine gelatin by measuring thermal characteristics using differential scanning calorimetry (DSC) and cooling curve method. Thermochim Acta. 2010;509:111–9.
Al-Saidi G, Rahman MS, Al-Alawi A, Guizani N. Thermal characteristics of gelatin extracted from shaari fish skin: effects of extraction conditions. J Therm Anal Calorim. 2011;104:593–603.
le Parlouër P. TG-DSC as a new method of investigation of polymers and resins. J Therm Anal. 1988;33:1085–90.
Riesen R, Vogel K, Schubnell M. DSC by theTGA/SDTA851e considering mass changes. J Therm Anal Calorim. 2001;64:243–52.
Xie W, Pan W-P. Thermal characterization of materials using evolved gas analysis. J Therm Anal Calorim. 2001;65:669–85.
Giron D. Applications of thermal analysis and coupled techniques in pharmaceutical industry. J Therm Anal Calorim. 2002;68:335–57.
TA Instruments, New Castle DE. Operator’s Guide: DSC high volume pan kit. PN 900826.001 Rev. H. Issued April 2003.
Acknowledgements
The authors are grateful to TA Instruments, New Castle, DE for generating the simultaneous DSC–TG data on calfskin gelatin using their Q600SDT instrument, as well as Kraft Foods Global Inc. for their support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mukherjee, I., Rosolen, M. Thermal transitions of gelatin evaluated using DSC sample pans of various seal integrities. J Therm Anal Calorim 114, 1161–1166 (2013). https://doi.org/10.1007/s10973-013-3166-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-013-3166-4