Abstract
Composites of high-density polyethylene (HDPE) and coffee dregs (COFD) were elaborated using four different types (integral, extracted, major size, and minor size) of COFD. The aim was to study the effects of particle size and soluble extraction over the properties of the HDPE. Four blends were made at the proportion of 90–10 % polymer-filler. The materials were evaluated through optical and scanning electron microscopy, differential scanning calorimetry and thermogravimetry/derivative thermogravimetry. The results showed that the integral COFD has a performance similar to the minor size one, and superior to the extracted. The composites degraded in two steps. The first one was in a temperature lower than the neat HDPE, but higher than the processing temperature of the polymer. The melting temperature and the degree of crystallinity of the composites resulted similar to the neat HDPE ones. In general, extraction and particle size of the COFD have little influence on the behavior of the HDPE. The results show that COFD can be used as filler in polymeric composites.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Both civil society and scientific community identify the urgent need to develop sustainable industrial alternatives. The economic model based on infinite growth is no longer viable in an environment of finite resources [1–3]. Thus, the development of biodegradable polymers, and polymeric alternatives to be used as replacement of natural materials, has been strongly encouraged [4–7]. From the perspective of life cycle analysis of plastics, it is necessary to adapt the way the players—involved in the production process—handle the finite natural resources used in the area. Proposals with “cradle-to-cradle” life cycle must be elaborated, since the raw materials of polymeric composites are also a finite natural resource (petroleum). Further, the environmental problems caused by their disposal are still a matter of concern without a solution compatible with the scale of the damages caused by them.
Within a group that embraces many of these placements, the field of polymers science and technology offers virtually an unlimited range of opportunities. Cost reduction and improvement of the industrialization of some products are factors that encourage the search for new polymeric materials. In addition, plastics have specific properties such as corrosion resistance, ease of molding, and they require less energy than metals for industrialization [8–10]. It is easy to find in the literature articles about the development of polymer composites reinforced with natural fibers, all aiming to improve the mechanical performance of the pure polymer. Further, due to their low-cost and large-scale production, polyolefins are the most frequent base-polymer of these composites. They are also the type of plastic present in larger volume as the municipal solid waste (MSW) in big cities. For this reason, polyolefins are often the target of researches involving recycling of polymers [11–13].
Based on the above information, the main aim of this study is to develop a composite of high-density polyethylene (HDPE) filled with coffee dregs (COFD). The central focus is to use the COFD—an organic waste present in large quantities as industrial and household waste, without commercial destination—as filler of polymeric composites. This study investigated the influence of particle size and the soluble fraction present in COFD, on the thermal properties of the HDPE. For this purpose, a pure polymer was used without any additives, to enhance possible effects of the filler on the characteristics of polymer.
Materials and methods
Materials
The materials used were HDPE QUATTOR® ES-60004 produced by UNIPOL® process, in the form of pure powder, having density of 0.960 g cm−3, and melt flow rate (MFR) of 0.35 g 10 min−1—donated by QUATTOR. The COFD were obtained through selective collection held at the IMA-UFRJ facilities. The integral COFD (COFD-I) was dried in an oven and separated by particle size analysis. The fractions of larger size (retained on 20 mesh sieve, COFD-MA) and smaller size (remaining at the bottom, passing through the 50 mesh sieve, COFD-ME) were used in the preparation of composites. The soluble were extracted by Soxhlet, using 20 g of integral COFD and 80 % formic acid as solvent, at a ratio of 1/6, for 12 h. The extracted COFD (COFD-E) was also used in the composites.
Composites preparation
The composites were prepared using the polymer/filler ratio of 90/10 wt%. It has also been processed as pure polymer at the same conditions, to compare the results with those of the composites. The HDPE and the four types of COFD were dried for 12 h in oven at 70 °C. The materials were prepared in an extruder AX Plastics Lab16 AX1626 model, with three heating zones, under the following processing parameters: first heating zone at 160 °C, second at 175 °C, third at 190 °C; and screw speed of 60 rpm. After the processing, the composites were pelletized and dried in an oven for 2 h at 100 °C. A small portion of each composite was ground in a knife mill Biovera Solab GL30 model, and the resulting powders were used to perform the TG/DTG and DSC analyses. The optical and scanning electron microscopy (SEM) were performed over laminates, obtained by compression molding in a Carver press at 150 °C, at 28 MPa, for 5 mins, and then cooled in a Carver press at 25 °C, at 35 MPa, for 5 mins.
Optical microscopy (OM)
Optical microscopy was performed in an optical microscope Olympus stereo SZH10 model, coupled with a camera Nikon COOLPIX 5400 model 5.0 megapixel. It was used at four times magnification.
Scanning electron microscopy
The scanning electron microscopy was performed over the cross section of cryogenically fractured samples, using a Jeol equipment model JSM-5610LV. The samples were previously metallized with a 300-nm layer of gold, using a Denton Vacuum Desk II equipment, model CARB ROD, series 40757.
Thermogravimetry/derivative thermogravimetry (TG/DTG)
The thermogravimetry/derivative thermogravimetry was performed using a TA equipment model Q500. The temperature range was from 30 to 700 °C, at a heating rate of 10 °C min−1, under nitrogen atmosphere. The mass loss, initial (T onset), maximum (T max), and final (T final) degradation temperatures were measured.
Differential scanning calorimetry
The differential scanning calorimetry (DSC) was performed using a Perkin Elmer equipment DSC7 model. The samples were analyzed under nitrogen atmosphere, according to the following cycles: in the first cycle, the sample was heated from 20 to 200 °C, at a heating rate of 10 °C min−1, leaving the material at 200 °C for 2 min; the second cycle was done using a cooling rate of 10 °C min−1, until 20 °C; in the third cycle, the sample was heated from 20 to 200 °C, at a heating rate of 10 °C min−1. The crystalline melting temperature (T m) and the degree of crystallinity (X c) of the HDPE were obtained considering the second heating curves. The X c was determined based on the ratio between the melting enthalpy (ΔH m) of the HDPE in the composite and the 100 % crystalline HDPE (290 J g−1), adjusted according to the content of polyolefin in the composite.
Results and discussion
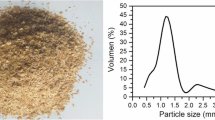
The OM analysis revealed a good dispersion of the COFD in the polymer. Figure 1 showed that the particle size of the four types of COFD is heterogeneous, with large variation in size even in those separated by size (COFD-MA and COFD-ME). The appearance differs from that expected for vegetable fibers (cylinders), probably as a result of the roasting that the material undergoes when industrialized. The SEM analysis (Fig. 2) showed more details on this morphology, revealing agglomerates of dendritic appearance, with very similar structures. The COFD-MA has greater spacing between the structures. The COFD-E showed a less-rounded shape of dendroids, compared to the ones in COFD-I. The presence of the filler in the composite is hard to see (Fig. 3), and apparently no change (shearing) has happened in particle size of COFD after the extrusion process.
In the thermogravimetric analysis of the composites, while observing the superposition of the curves (Fig. 4), there was a first step of degradation around 250 °C, followed by another step around 380–390 °C (typical onset of the HDPE [14, 15]). The HDPE/COFD-I behavior was similar to HDPE/COFD-ME. The thermal performance of HDPE/COFD-E resembled the HDPE/COFD-MA—both showing greater mass loss than HDPE/COFD-I and HDPE/COFD-ME between 250 and 450 °C. The effect of the extraction on the COFD was a decrease in the thermal stability of the composite, indicating that the material removed (possibly lignin) functions as a thermal protection material. This same decrease was observed in HDPE/COFD-MA curve, which has probably retained portions of the noncrystallized HDPE in the COFD-MA larger voids. These portions may have degraded before. In general, the presence of COFD in the composites did not cause degradation of the material in the temperature range commonly used for thermoforming articles (180–200 °C). The T max and T final remained very close to those of neat HDPE.
Regarding the DSC curves, they revealed that there was no significant variation in the T m of the HDPE in the composites. The peaks resulted also similar, but with the melting enthalpy lower (from 12 to 17 %) than the pure polymer one, indicating an interference of the filler in the crystalline packing of the polymer. The composite HDPE/COFD-MA showed a significant reduction (15 %) of the degree of crystallinity (X c), as shown in Table 1. This could be ascribed to the penetration of the molten polymer into the voids among the COFD-MA dendroids. It might have hindered the diffusion of the molecules to the centers of crystallization. The crystallization temperature (T c) of the composite remained practically the same of the HDPE.
Conclusions
Despite the appearance of a first stage of degradation, the composites showed thermal resistance similar to that of HDPE in the second step of degradation, with T onset and T final being nearly equal. No degradation happened in the processing temperature range of HDPE. The composites HDPE/COFD-I and HDPE/COFD-ME degraded in a similar manner, and at higher temperatures than those of HDPE/COFD-E and HDPE/COFD-MA. The thermal behavior of HDPE/COFD-E is quite similar to HDPE/COFD MA. Both materials present greater mass loss than HDPE/COFD-I and HDPE/COFD-ME ones. The T m of the composites resulted was almost equal to that of the neat polymer and, except for composite COFD-MA, there was a little reduction in the value of the X c.
The COFD-I resembles COFD ME in morphology and thermal performance, and has better results than COFD-E in terms of thermal stability and influence on the crystallinity of the HDPE. The study indicates that COFD is an interesting filler material for polymeric composites, since it does not need the soluble extracting process and the use of small-sized particles. This is an advantage in terms of processing, because it skips pretreatment steps and/or size separation; allows COFD—a natural fiber that has been treated for centuries as waste, discarded after a prewash in boiling water (soluble extract); and already presents a reduced particle size—to be used as a new input in the universe of natural fiber composites.
References
Solow RM. The economics of resources or the resources of economics. Am Econ Rev. 1974;64:1–14. Papers and Proceedings of the Eighty-sixth Annual Meeting of the American Economic Association. (May, 1974). http://links.jstor.org/sici?sici=0002-8282%28197405%2964%3A2%3C1%3ATEOROT%3E2.0.CO%3B2-4.
Penteado H. A mudança dos paradigmas e o mito do crescimento. J Valor. São Paulo, 19 abr. 2010. http://www.valoronline.com.br/impresso/investimentos/119/115591/a-mudanca-dos-paradigmas-e-o-mito-do-crescimento. Accessed 15 Feb 2012.
Brown LR. Eco-economy: building an economy for the earth. 1st ed. New York: W. W. Norton & Co.; 2001.
Satyanarayana KG, Arizaga GGC, Wypych F. Biodegradable composites based on lignocellulosic fibers: an overview. Prog Polym Sci. 2009;34:982–1021.
Parente AP. Elementos estruturais de plástico reciclado. Dissertation 2006. 142 p. Dissertação (Mestrado em Engenharia de Estruturas)—Escola de Engenharia de São Carlos, Universidade de São Paulo, São Carlos, SP, 2006. Orientador Prof. Libânio Miranda Pinheiro.
Mark HF, Atlas SM. Polymers as building materials. Annu Rev Mater Sci. 1976;6:1–33.
Herrera-Franco PJ, Valadez-González A. A study of the mechanical properties of short natural-fiber reinforced composites. Composites Part B. 2005;36:597–608.
Taj S, Munawar MA, Khan S. Natural fiber-reinforced polymer composites. Proc Pakistan Acad Sci. 2007;44(2):129–44.
Candian LM. Estudo do polietileno de alta densidade reciclado para uso em elementos estruturais. 2007; 153 p. Dissertation (Mestrado em Engenharia de Estruturas)—Escola de Engenharia de São Carlos, Universidade de São Paulo, São Carlos, SP, 2007. Orientador Prof. Dr. Antônio Alves Dias.
Lei Y, Wu Q, Yao F, Xu Y. Preparation and properties of recycled HDPE/natural fiber composites. Composites Part A. 2007;38:1664–74.
Moraes SRP, Oliveira ARL, Souza JF, Alves JD. Avaliação de polímeros termoplásticos recicláveis como materiais componentes de telhas e tijolos. Goiânia: Enciclopédia Biosfera/Centro Científico Conhecer, 2010. http://www.conhecer.org.br/enciclop/2010c/avaliacao%20de%20polimeros.pdf. Accessed Jun 2011.
Mendes LC, Cestari SP. Printability of HDPE/natural fiber composites with high content of cellulosic industrial waste. Mater Sci Appl. 2011;2(9):1331–9.
Aji IS, Zainudin ES, Khalina A, Sapuan SM, Khairul MD. Thermal property determination of hybridized kenaf/PALF reinforced HDPE composite by thermogravimetric analysis. J Therm Anal Calorim. 2011;. doi:10.1007/s10973-011-1807-z.
Kim H-S, Yang H-S, Kim H-J, Park H-J. Thermogravimetric analysis of rice husk flour filled thermoplastic polymer composites. J Therm Anal Calorim. 2006;76(2):395–404.
Panar M, Epstein BN. Multicomponent polymeric engineering materials. Science. 1984;22:642–6.
Acknowledgments
The authors extend their thanks to the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and to the Fundação Coordenação do Aperfeiçoamento de Pessoal de Nível Superior (CAPES), for supporting this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sibele Piedade, C., Luis Claudio, M. Thermal properties and morphology of high-density polyethylene filled with coffee dregs. J Therm Anal Calorim 114, 1–4 (2013). https://doi.org/10.1007/s10973-013-3121-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-013-3121-4