Abstract
The hydration of cellulose, chitosan, schizophyllan, hyaluronan, and carboxymethyl cellulose was studied using differential scanning calorimetry (DSC). In the first part, the classical freezing/thawing approach was used to determine the amount of non-freezing water. The inconsistency in enthalpies obtained during crystallization and melting of freezable water was discussed with respect to the DSC experimental conditions. Our interpretation questions the recent conclusions about competitive processes occurring during melting which are hypothesized to influence the determined melting enthalpy. In the second part, the hydration and drying were studied using the evaporation enthalpy of water. The dry mass normalized dependency of vaporization enthalpy on water content confirmed an abrupt break at low water content in hyaluronan sample which was attributed to the sudden appearance of a parallel process taking part during the drying. The rest of polysaccharide samples showed only a linear decrease in evaporation enthalpy. The renormalization of enthalpies by the water content revealed the increase in evaporation enthalpy with decreasing water content in most samples which was ascribed to the strong interaction between polysaccharide and water. The exceptions were carboxymethyl cellulose which showed a decrease in evaporation enthalpy. This indicates the existence of a simultaneous process occurring during drying, but unlike in hyaluronan, the processes do not appear abruptly but accompany the evaporation in the wide concentration range. Comparison of determined hydration numbers showed that part of non-freezing water in hyaluronan is not bound to sorption sites but occurs presumably in small temporary pores. In contrast, water-soluble schizophyllan forms temporary pores as well but presumably with higher dimension and the non-freezing water is formed mostly by water molecules interacting with sorption sites.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Native biopolymers and biopolymer-based materials have been long time studied with respect to their environment-friendly character and adjustable properties [1, 2]. Polysaccharides have been widely investigated for their specific interactions with water due to its strong influence on their physical structure [3–6]. Physical structure of some polysaccharides reversibly changes in the presence of even a trace amount of water. As a results, new ordered structures stabilized by water molecules can be formed [7, 8] while it is assumed that their character is determined by the hydration status of present functional groups [9].
The interactions between water and various polymers have been studied using thermal [8, 10], NMR [11] dielectrical [12] and spectroscopic techniques [4, 13], viscometry [14], and/or by means of computer simulations [15]. Among thermoanalytical techniques differential scanning calorimetry (DSC) found wide popularity [10, 11].
The traditional way of classification of hydration water using DSC is based on the freezing/thawing experiments in which the difference in physical properties of freezable water in form of ice and non-freezing water is investigated. In brief, non-freezing water (NFW) is the pool of water molecules present in the close proximity of a polysaccharide (restricted in pores or bound by polar groups) and does not exhibit any crystallization and melting upon cooling and heating, respectively. The increasing water content brings about the formation of new layers in which water molecules are less restricted and thus it can freeze. This layer is traditionally called freezing bound water. The structure of ice formed in this layer differs from the ice formed from the bulk or non-restricted water. Those properties are, among others, significantly low melting enthalpy, freezing point depression, supercooling effect and strong dependence on thermal history [16, 17]. As a result, the freezing bound water can be distinguished from free (bulk) water although both fractions are considered as freezable water. Recent results indicate that existence of non-freezing and freezing bound water can be caused to some extend by pores and cavities formed by dried and semi-dried biopolymers [18], which is in accordance with earlier suggestion [19]. Based on difference between enthalpy of ice formation and melting, Gemmei-Ide et al. [20] tried to demonstrate that the hydration of (bio)polymers studied by DSC is in principle biased by the existence of phase transitions such as condensation, deposition, sublimation, and vaporization which during the heating run could be detected by FTIR but it could not be detected by DSC.
Alternative DSC approaches are based on determination of enthalpy of water vaporization from semi-diluted polysaccharide solutions. Those experiments provided additional information regarding the state of water hydration in hyaluronan, [18, 21], cellulose fibers [7, 22] and chitosan [23]. In case of hyaluronan, it has been demonstrated that during drying, at certain water content, an additional process to evaporation occurred. This process compensated slightly the evaporation enthalpy [18] and it was attributed to the formation of weak interactions stabilizing hyaluronan structure. Further, authors concluded that this process is potentially exploitable in development of physically modified biopolymer-based materials. However, the origin of the process is not clear yet and the number of biopolymers tested by this approach is still limited. In case of cellulose fibers [24], the evaporation enthalpy was normalized by water mass which showed an increasing trend in dependency on water content. The authors concluded that the increase is caused by the increased energy of interaction between hydrophilic surface and water molecules and pointed out the similarity between the concentration at which the increase started and the amount of non-freezing water determined by freezing/thawing experiments in their previous work [22].
The first aim of this work is to explain the differences between enthalpies of crystallization and melting of freezable water in polysaccharides to test the conclusions reported in Ref. [20]. The second aim of this work is to study the water evaporation from several polysaccharides to understand to processes occurring during water evaporation from individual polysaccharides, to the origin of the compensation process observed in hyaluronan and if such a process is typical for other polysaccharides as well. In this study, following polysaccharides were used: chitosan, a biodegradable hydrophilic biopolymer consisting of β-(1-4)-linked d-glucosamine (deacetylated) and N-acetyl-d-glucosamine (acetylated) units, insoluble but swelling in water [25]; schizophyllan, a neutral extracellular polysaccharide consisting of β-1,3-d-glucose backbone with β-1,6-d-glucose side chain for every three main chain residues [26], soluble in water [27]; hyaluronan, sodium salt, a linear, water-soluble, unbranched, high molecular weight extracellular matrix polar polysaccharide composed of repeating disaccharide units which consist of N-acetyl-d-glucosamine and d-glucuronic acid linked by a β 1-4 glycosidic bond [28], cellulose, a linear polysaccharide, composed of repeating 1,4-β-d-glucopyronose units, insoluble but swelling in water [29] and carboxymethyl cellulose, a sodium salt of anionic water-soluble natural polymer derivative of cellulose [30].
Materials and methods
Polysaccharides
Specification of investigated polysaccharides is as follows: bacterial hyaluronan (HYA) with molecular weight 73 kDa, specifically its Na+ form, and schizophyllan (SCHIZ) (batch number 241108) were kindly provided by CPN Company (Dolni Dobrouc, Czech Republic). Carboxymethyl cellulose (CMC) in Na+ forms with molecular weights 90 kDa (degree of substitution; DS = 0.7) and 700 kDa (DS = 0.9) were purchased by Sigma-Aldrich (Germany). Chitosan (CHIT), medium molecular weight (190–310 kDa) with the degree of deacetylation (DD) = 0.75–0.85 and microgranular cellulose (CELL) (batch 126K0125) were purchased by the same supplier. All samples were used as received.
Preparation of biopolymer/water systems
In case of freezing/thawing experiments, samples of ~2–3 mg were placed into aluminum sample pans (Perkin Elmer, 50 μL) and Milli-Q water was added. Excess of water was allowed to evaporate slowly at room temperature until the desired water content (controlled by balances) was obtained. The pans were subsequently hermetically sealed and left to equilibrate at room temperature for 48 h. The pans mass were determined again before and after the experiment to insure that no mass loss had occurred.
Samples for evaporation experiments were prepared in the same way, but different type of the aluminum pans was used (TA Instruments, Tzero® technology).
Water concentration in this work is defined as water fraction related to dry mass (W c), i.e., W c = grams of water/grams of dry sample (g g−1) [31].
Thermal analysis
Freezing/thawing experiments were carried out by differential scanning calorimetry (DSC) device Perkin Elmer Pyris 1, equipped with a cooling device (Perkin Elmer Intracooler 2P). Prior to the analysis, the temperature and enthalpy determined by the device was calibrated by standards (phenyl 2-hydroxybenzoate, In, Sn, and Pb) using a standard procedure in which onset point and peak area of melting were taken into account [32]. All freezing/thawing experiments were carried out using following temperature program: start at 30 °C; cooling from 30 °C to −70 °C at 2 °C min−1; isothermal at −70 °C for 1 min; heating from −70 °C to 30 °C at 7 °C min−1. Flow rate of purge nitrogen atmosphere was 30 mL min−1.
Evaporation experiments were carried out using the TA Instruments DSC Q200 equipped with a cooling accessory RCS90 and assessed by the TA Universal Analysis 2000 software. The system was previously calibrated by In standard for temperature and enthalpy and verified by phenyl 2-hydroxybenzoate, Sn and Pb standards. The following thermal protocol was used for measurement of evaporation enthalpy: equilibration at 27 °C; cooling from 27 °C to −40 °C at 10 °C min−1; isothermal at −40 °C for 2 min; heating from −40 °C to 250 °C at 3 °C min−1. At −40 °C, the flow rate of nitrogen was switched from 50 mL min−1 to 5 mL min−1. In contrast to earlier experiments [21], in this case, prior to the measurement the hermetically sealed pan (Tzero®) was perforated only partially (1 hole) to have higher pressure inside the pan during the evaporation in contrast to Ref. [21]. Selected concentrations were tested for all samples in triplicate and the deviation never exceeded 3.5 % of measured enthalpies and ±0.5 °C for the determined temperature.
Thermogravimetry (TA Instruments, Q500) was used to determine the equilibrium moisture content in purchased polysaccharides as a mass loss in the temperature interval from 25 to ~180 °C under dynamic atmosphere of nitrogen 40 mL min−1 to precisely define the W c values (data not reported).
Results and discussion
Freezing/thawing experiments
The illustration of DSC experiments, i.e., freezing/thawing and evaporation of water from chitosan are depicted in Fig. 1. Transitions A and B correspond to freezing and thawing experiments, respectively. The transition A is connected with crystallization of freezable water, B shows the melting of ice formed during the A stage. The broad peak C represents evaporation of water from the polysaccharide (conducted in separate experiment).
Representative DSC cooling and heating curves for the carboxymethyl cellulose mixed with water at various W c (the experiments “A” and “B”) are shown in Fig. 2. Figure 2a reports the example of ice melting in carboxymethyl cellulose with increasing W c. It can be seen that at W c = 0.9 no peak occurred which indicates that all the water molecules occur as non-freezing water [21]. It is worth to mention that the sample mass was usually at least 2 mg which means that amount of water in the pan was around 0.95 mg. The melting enthalpy of pure water is 333 J g−1, i.e., in case of no interaction between the polysaccharide and water, the resulting melting enthalpy would be 316 mJ which is value far above the sensitivity of DSC device used in this work. Therefore the absence of the peak cannot be considered as a sensitivity issue. At concentration of water W c = 1.06 a weak exothermal process appeared around −40 °C connected with (re)crystallization of freezing bound water followed by melting of freezable water pool around −11 °C. Increase in W c caused the disappearance of exotherm and only melting could be seen with increasing onset point. Figure 2b reports the results of crystallization of water in carboxymethyl cellulose/water mixture. Unlike the melting curves reported in Fig. 2a, exothermal peak became visible at W c = 1.18 (onset is around −27 °C); these values were shifted irregularly to higher values with increasing W c. At lower W c, the peak was slightly split; while with increasing W c, crystallization peak is narrower and its position does not change significantly. This observation is in line with results of Faroongsarng et al. [33] who reported two DSC peaks during crystallization and melting of absorbed water on carboxymethyl cellulose and assigned them as freezing bound and free water pools.
The determined enthalpies of melting and crystallization (peak areas) were first normalized dividing them by the mass of the dry sample and then plotted against their respective W c [21, 31]. Figure 3 reports the dependency of enthalpy of water crystallization and melting normalized to the mass of dry sample plotted against the W c obtained for chitosan/water mixtures. Linear dependences were obtained for all polysaccharides measured in this study. The linear behavior is typical for hydrophilic biopolymers (e.g., [34]) while it differs in comparison with mixtures of amphiphilic and hydrophobic molecules for example humic acids [35].
The content of non-freezing water was obtained for both melting (NFW) and for crystallization experiments (NFWc). The dependency was linearized (y = ax + b) and the hydration number was determined as the concentration at which the first measurable enthalpy appeared, i.e., the interception at y = 0 [31]. The calculated values of NFW and parameters of linearization are listed in Table 1. In contrast, dependencies of crystallization enthalpy showed linear trend only at lower W c and smaller or larger deviation from linear trend at higher values of W c (as reported in Fig. 3). Thus, the linear regression was carried out only for data obtained at lower W c. Since the break in dependency appeared at similar W c in all samples it can be assumed that the non-linearity at higher W c is caused by limitation of device used for the measurement, i.e., it is not able to compensate effectively the heat released by the sudden crystallization of water in the sample associated with supercooling effect.
The data reported in Table 1 show the inconsistency between NFW and NFWc. Their difference indicates the significant specificity of water crystallization and melting in those particular polysaccharides which will be discussed later.
Evaporation experiments
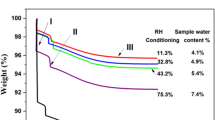
Evaporation of water from a polysaccharide is an alternative approach to characterize the hydration of biopolymers by means of DSC. The method suggested by Prusova et al. [21] was used in this work. The determined enthalpies of evaporation (experiment “C” in Fig. 1) were normalized by the dry sample masses and then plotted against the respective W c. Figure 4 shows the comparison of results of evaporation experiments for Na+ form of HYA obtained in this work and recently in [21].
Enthalpy of water evaporation from hyaluronan normalized by dry mass in dependency on water content. The black points correspond to the data reported in Ref. [21]
The linear dependency of normalized evaporation enthalpy on W c showed a break around W c = 0.18 for higher pressure and 0.34 for ambient pressure (data were reported in [21]). These differences indicate the pressure-dependency of the process causing the disruption in the linear decrease. As already reported, this break was attributed to a competitive process which occurs during water evaporation from HYA and which is associated with heat evolution. Such heat evolution has been attributed to formation of intermolecular interactions in dry HYA [18, 21]. Recent findings indicate that this process might be associated with the plasticization point of HYA and the compensation process is thus caused partially by the sudden decrease in heat capacity of the HYA (Prusova and Kucerik, unpublished results). Figure 4 shows the shift of the break to lower W c at higher pressure then applied by Prusova et al. and confirms the previous statements [18, 21] about the importance of drying conditions on the resulted hyaluronan structure and a great potential for influencing of HYA physical structures by their manipulation.
Unlike the hyaluronan, the other polysaccharides investigated in this work did not show such a break (see the examples reported in Fig. 5). Instead, the dependencies had a linear behavior in the whole range of concentrations. A possible explanation of this difference between hyaluronan and other polysaccharides can be found in the conformation and molecular movement of HYA segments. HYA was described as crowded random coil molecules in liquid state, but, in solid state, it is predominantly a single helical conformation containing three disaccharides per helical turn, stabilized by H-bonds linking adjacent sugar residues across both glycosidic linkages [36]. It seems that two types of glycosidic bonds connecting two different glycosidic monomers grant to the HYA physical structure significant flexibility and mobility of chains associated with a range of thermodynamically stable states [37].
Park et al. [24] suggested the normalization of evaporation enthalpy by water mass which improves the information value obtained from the evaporation experiments. Figure 6 shows the examples of those calculations for CMC 90 and 700 kDa and for chitosan and schizophyllan. In comparison with pure water, both CMC samples showed a decrease in evaporation enthalpy at the low W c. In contrast, chitosan and schizophyllan (Fig. 6), showed an increase which is in accordance with results of Park et al. [24] who observed the increasing value for hydrophilic cellulose samples and constant value for hydrophobic surfaces. The increase in enthalpy was observed for cellulose in this study as well (not reported). The enthalpy of vaporization for pure water is 2.5, 2.41, and 2.31 kJ g−1 for 0, 40, and 80 °C, respectively [38]. The energy of binding between water molecules and the sorption sites is higher than the energy which holds the molecules of pure water [24]. Therefore, in case of carboxymethyl cellulose, the evaporation enthalpy is decreased by a simultaneous process but unlike in case of hyaluronan the process does not appear abruptly. Instead, it takes part in the whole range of investigated concentrations and thus it could not be detected by the approach reported in Figs. 4 and 5. Similarly, as in case of hyaluronan, the character of this process is currently unknown. However, it can be rejected the hypothesis that it is caused by sodium cation because the break appeared also in H+ form of hyaluronan [21]. Closer analysis of determined enthalpies suggests that lower molecular weight of carboxymethyl cellulose has slightly larger influence on the decrease of evaporation enthalpy, which is similar to behavior of hyaluronan [21] and can be related to the movement of carboxymethyl cellulose segments during water elimination. Evaporation enthalpy of water from schizophyllan is rather complicated, the enthalpy increased first at W c = 1.1 then decreased and then again increased at around W c = 0.38 indicating a two-step process. In case of hyaluronan, the tendency of enthalpy related to water content increased similarly as in case of chitosan. This is in agreement with the recent study [18] which showed that despite the fact that a compensation process takes part, the evaporation enthalpy of water from hyaluronan is still higher than evaporation enthalpy of pure water.
Hydration numbers were obtained from evaporation experiments in the same way as suggested by Park et al. [24] In this case, the hydration number corresponded to the concentration at which the increase in vaporization enthalpy differed from the enthalpy of vaporization of pure water mentioned above. Since the enthalpies obtained from carboxymethyl cellulose were lower in the whole concentration range, the hydration numbers could not be obtained by this way. The approximate values are listed in Table 2 and reveal specific behavior of polysaccharides during drying.
Schizophyllan and hyaluronan are water-soluble polysaccharides and showed similar properties and hydration numbers during drying. It can be assumed that the most important mechanism is desorption of water from sorption sites or temporary micropores. In contrast, chitosan is water insoluble swelling compound and water is presumably restricted by cross-linked molecular network. Therefore, the enthalpy of evaporation is influenced by the diffusion and osmotic pressure. In case of cellulose, insoluble slightly swelling hydrophilic polysaccharide, the drying mechanisms is already well described and involves desorption from sorption sites as well as the capillary effect [22, 24]. The hydration of cellulose is influenced by its crystallinity which is in case of granular cellulose used in this work more than 50 % and causes the low hydration degree since the crystalline domains cannot be easily hydrated.
Comparison of determined hydration numbers
Three different approaches used in this work brought different results as it can be seen in Tables 1 and 2. The freezing/thawing experiments were carried out under similar temperature conditions but also in this case the results were different. As already mentioned, Gemmei-Ide et al. [20] concluded that the DSC freezing/thawing experiment is biased by several phase transitions accompanying melting run and which cannot be detected by DSC. In this work, the differences between enthalpies of crystallization and melting were determined as well. Figure 7a, b shows the comparison of hydration numbers obtained from melting and crystallization reported in Tables 1 and 2 related to the gram and to molecular mass of the monosaccharide unit, respectively.
As indicated in Fig. 7, the dependence between hydration numbers obtained from cooling and thawing experiments is linear. The separate freezing/thawing DSC experiments carried out in triplicate with pure water (not shown) gave following results: melting enthalpy 332.2 ± 0.5 J g−1, cooling enthalpy 294.0 ± 2.1 J g−1, their ratio is around 1.13 similarly as the parameter of linear regression reported in Fig. 7a, b. The ratio obtained for pure water slightly increases with increasing heating and cooling rate and water amount in the pan. The disproportion between crystallization and melting peak area was also confirmed using two other DSC devices from different providers. Since calibration standards (In and Sn) gave the same results for cooling and heating runs, the difference in enthalpy of crystallization can be therefore attributed to the water properties. First, the difference in enthalpies is big enough to be detected by DSC and processes such as evaporation and sublimation should be detected as a shoulder on the melting endotherm which is not always the case (see Fig. 3). Next, the suggested processes of sublimation/evaporation followed by the deposition should be also seen even at the concentration below the determined non-freezing water concentration since water is already present in polymer matrix and DSC is sensitive to detect such enthalpies since they are relatively high (i.e., 45 and 51 kJ mol−1 for evaporation and sublimation of pure water, respectively). Further, the experimental conditions for FTIR spectroscopy which was used to detect those processes [20] are not perfectly described, but they are probably different in comparison with DSC where the sample is measured in a small hermetically sealed pan. Last but not least, the supercooling effect takes part during ice formation (see Fig. 1), i.e., formation of hexagonal ice is restricted by water diffusion and insufficient crystallization heat compensation. In restricted geometries, depending on the size and shape, the formation of glassy water, imperfect crystals, or cubic ice is often [39]. Figure 1 reports the freezing/thawing experiment and it is clear, that the only part in which the additional enthalpy can evolve is the part where is the heating switched into cooling regime. In other words, the difference between melting and crystallization enthalpy is “hidden” in the part of the signal which is affected by changing of the sign from negative to positive and which is not usually taken into account.
The hypothesis about the water restriction is supported by the data presented in Fig. 8 where the ratio between melting and crystallization enthalpy is plotted as a function of water content in schizophyllan/water samples. It can be seen that increasing water content causes the increase in the ratio between melting and crystallization enthalpies. Other polysaccharides gave similar results. Second argument supporting our hypothesis is the absence of crystallization peak at the W c at which the melting can already be seen, e.g., Fig. 3, W c = 1.06. It demonstrates the restriction of water to crystallize, i.e., cooling run where no crystallization peak takes place is followed by the heating run in which the ice “appears” and melts. As a result, the melting enthalpy can be used for determination of NFW but the difference between NFW and NFWc reflects the polymer/water interactions and polymer structural properties.
Park et al. [24] reported, that hydration number obtained by evaporation are comparable with non-freezing water. In fact, some difference might be expected due to different temperatures ranges of both experiments. Comparison of values reported in Tables 1 and 2 seem to confirm this conclusion only in some cases. Schizophyllan showed the two-step evaporation which indicates that water was evaporated first from pores and in the second step from the adsorption sites. This assumption confirms the non-freezing water content (Table 1) which agrees with the second increase in evaporation enthalpy (Fig. 6; Table 2). That means that all the non-freezing water is adsorbed on sorption sites of schizophyllan. In contrast, hyaluronan, other water-soluble polysaccharide, showed higher content of non-freezing water (Table 1) in comparison with hydration number determined by evaporation. This indicates that in hyaluronan the non-freezing water is formed by water which is both bound by adsorption sites and restricted by temporary pores. Therefore, the pore size of hyaluronan structure is necessarily lower than in schizophyllan which explains why this water fraction cannot freeze. In contrast, in schizophyllan, the pores are big enough to allow freezing of restricted water. The comparison of hydration numbers of chitosan showed smaller value of non-freezing water (Table 1) and larger value of hydration determined by evaporation (Table 2). This confirms the hypothesis about the influence of pore size on the character of water in the non-freezing water layer. The decrease in evaporation enthalpy as seen in schizophyllan did not occur which is caused by “permanent” character of cross-linking resulting in more rigid structure of chitosan and its high affinity to water [40].
References
Cavallaro G, Donato DI, Lazzara G, Milioto S. Films of halloysite nanotubes sandwiched between two layers of biopolymer: from the morphology to the dielectric, thermal, transparency, and wettability properties. J Phys Chem C. 2011;115:20491–8.
Cavallaro G, Lazzara G, Milioto S. Dispersions of nanoclays of different shapes into aqueous and solid biopolymeric matrices. Extended physicochemical study. Langmuir. 2011;27:1158–67.
Dammström S, Salmén L, Gatenholm P. The effect of moisture on the dynamical mechanical properties of bacterial cellulose/glucuronoxylan nanocomposites. Polymer. 2005;46:10364–71.
Gocho H, Shimizu H, Tanioka A, Chou TJ, Nakajima T. Effect of polymer chain end on sorption isotherm of water by chitosan. Carbohydr Polym. 2000;41:87–90.
Blicharska B, Peemoeller H, Witek M. Hydration water dynamics in biopolymers from NMR relaxation in the rotating frame. J Magn Reson. 2010;207:287–93.
Mracek A, Benesova K, Minarik A, Urban P, Lapcik L. The diffusion process of sodium hyaluronate (Na-HA) and Na-HA-n-alkyl derivatives films swelling. J Biomed Mater Res Part A. 2007;83A(1):184–90.
Hatakeyama T, Nakamura K, Hatakeyama H. Vaporization of bound water associated with cellulose fibers. Thermochim Acta. 2000;352–353:233–9.
Hatakeyama H, Hatakeyama T. Interaction between water and hydrophilic polymers. Thermochim Acta. 1998;308:3–22.
Hatakeyama T, Hatakeyama H, Nakamura K. Non-freezing water content of mono- and divalent cation salts of polyelectrolyte–water systems studied by DSC. Thermochim Acta. 1995;253:137–48.
Takahashi M, Hatakeyama T, Hatakeyama H. Phenomenological theory describing the behaviour of non-freezing water in structure formation process of polysaccharide aqueous solutions. Carbohydr Polym. 2000;41:91–5.
Průšová A, Conte P, Kučerík J, Alonzo G. Dynamics of hyaluronan aqueous solutions as assessed by fast field cycling NMR relaxometry. Anal Bioanal Chem. 2010;397:3023–8.
Hunger J, Bernecker A, Bakker HJ, Bonn M, Richter R. Hydration dynamics of hyaluronan and dextran. Biophys J. 2012;103:L10–L12.
Maréchal Y, Milas M, Rinaudo M. Hydration of hyaluronan polysaccharide observed by IR spectrometry. III. Structure and mechanism of hydration. Biopolymers. 2003;72:162–73.
Mracek A, Varhanikova J, Lehocky M, Grundelova L, Pokopcova A, Velebny V. The influence of Hofmeister series ions on hyaluronan swelling and viscosity. Molecules. 2008;13(5):1025–34.
Mazeau K, Rinaudo M. The prediction of the characteristics of some polysaccharides from molecular modeling. Comparison with effective behavior. Food Hydrocolloid. 2004;18:885–98.
Takigami S, Takigami M, Phillips GO. Hydration characteristics of the cross-linked hyaluronan derivative hylan. Carbohydr Polym. 1993;22:153–60.
Takigami S, Takigami M, Phillips GO. Effect of preparation method on the hydration characteristics of hylan and comparison with another highly cross-linked polysaccharide, gum arabic. Carbohydr Polym. 1995;26:11–8.
Kučerík J, Průšová A, Rotaru A, Flimel K, Janeček J, Conte P. DSC study on hyaluronan drying and hydration. Thermochim Acta. 2011;523(1–2):245–9.
Belton PS. NMR and the mobility of water in polysaccharide gels. Int J Biol Macromol. 1997;21:81–8.
Gemmei-Ide M, Ohya A, Kitano H. Thermally latent water in a polymer matrix. J Phys Chem B. 2010;114:4310–2.
Prusova A, Smejkalova D, Chytil M, Velebny V, Kucerik J. An alternative DSC approach to study hydration of hyaluronan. Carbohydr Polym. 2010;82(2):498–503.
Park S, Venditti RA, Jameel H, Pawlak JJ. Changes in pore size distribution during the drying of cellulose fibers as measured by differential scanning calorimetry. Carbohydr Polym. 2006;66(1):97–103.
Prado JR, Vyazovkin S. Activation energies of water vaporization from the bulk and from laponite, montmorillonite, and chitosan powders. Thermochim Acta. 2011;524(1–2):197–201.
Park S, Venditti RA, Jameel H, Pawlak JJ. Studies of the heat of vaporization of water associated with cellulose fibers characterized by thermal analysis. Cellulose. 2007;14(3):195–204.
Ghosh A, Ali MA, Walls R. Modification of microstructural morphology and physical performance of chitosan films. Int J Biol Macromol. 2010;46:179–86.
Nishinari K, Takahashi R. Interaction in polysaccharide solutions and gels. Curr Opin Colloid Interface Sci. 2003;8:396–400.
Kony DB, Damm W, Stoll S, van Gunsteren WF, Hűnenberger PH. Explicit-solvent molecular dynamics simulations of the polysaccharide schizophyllan in water. Biophys J. 2007;93:442–55.
Robert L, Robert A-M, Renard G. Biological effects of hyaluronan in connective tissues, eye, skin, venous wall. Role in aging. Pathol Biol. 2010;58:187–98.
Nikolakakis I, Tsarvouli K, Malamataris S. Water retention and drainage in different brands of microcrystalline cellulose: effect of measuring conditions. Eur J Pharm Biopharm. 2006;63:278–87.
Liu P, Zhai M, Li J, Peng J, Wu J. Radiation preparation and swelling behavior of sodium carboxymethyl cellulose hydrogels. Radiat Phys Chem. 2002;63:525–8.
Liu J, Cowman MK. Thermal analysis of semi-dilute hyaluronan solutions. J Therm Anal Calorim. 2000;59:547–57.
Della Gatta G, Richardson MJ, Sarge SM, Stolen S. Standards, calibration, and guidelines in microcalorimetry—Part 2 calibration standards for differential scanning calorimetry—(IUPAC Technical Report). Pure Appl Chem. 2006;78:1455–76.
Faroongsarng D, Sukonrat P. Thermal behavior of water in the selected starch- and cellulose-based polymeric hydrogels. Int J Pharm. 2008;352:152–8.
Qu X, Wirsén A, Albertsson AC. Novel pH-sensitive chitosan hydrogels: swelling behavior and states of water. Polymer. 2000;41:4589–98.
Kučerík J, Bursáková P, Průšová A, Grebíková L, Schaumann GE. Hydration of humic and fulvic acids studied by DSC. J Therm Anal Calorim. 2012;110:451–9.
Cowman MK, Matsuoka S. Experimental approaches to hyaluronan structure. Carbohydr Res. 2005;340:791–809.
Hargittai I, Hargittai M. Molecular structure of hyaluronan: an introduction. Struct Chem. 2008;19:697–717.
Marsh KN. Recommended reference materials for the realization of physicochemical properties. Oxford: Blackwell; 1987.
Kohl I, Mayer E, Hallbrucker A. The glassy water-cubic ice system: a comparative study by X-ray diffraction and differential scanning calorimetry. Phys Chem Chem Phys. 2000;2:1579–86.
Kerch G, Glonin A, Zicans J, Meri RM. A DSC study of the effect of ascorbic acid on bound water content and distribution in chitosan-enriched bread rolls during storage. J Therm Anal Calorim. 2012;108:73–8.
Acknowledgements
This work was financially supported by the Ministry of Education, Youth and Sport of the Czech Republic project no. 0021630501. Authors would like to thank Dr. Vladimir Velebný from CPN Company, Dolní Dobrouč, Czech Republic for providing hyaluronan and schizophyllan samples and to Ms. Alena Prusova for the help with evaporation experiments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mlčoch, T., Kučerík, J. Hydration and drying of various polysaccharides studied using DSC. J Therm Anal Calorim 113, 1177–1185 (2013). https://doi.org/10.1007/s10973-013-2946-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-013-2946-1