Abstract
The substitution of Sb with As in the NiSbS intermetallic compound was studied in the framework of evaluating a possible increase of the thermoelectric properties. Different NiSb1−xAsxS samples were synthesized with increasing amounts of As (0 < x < 0.66) employing a simple synthetic route using a muffle furnace. Scanning electron microscopy equipped with energy-dispersive X-ray spectroscopy was used to investigate the microstructure. X-ray powder diffraction techniques were employed in order to study the possible existence of a solid solution between NiSbS and NiAsS compounds, as well as to identify the crystal structure and determine the lattice parameters. All compounds were found to crystallise with the NiSbS prototype (cP12-P213), with lattice parameters varying from a = 0.59341(7) nm (x = 0) to a = 0.56849(6) nm (x = 1). Good agreement with Vegard’s law was evidenced. Thermal measurements on NiSb1−xAsxS samples were carried out using DTA instruments to evaluate the thermal stability and the melting temperatures.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
For years, the high dependence of society on fossil fuels has lead to political crisis and global climate change. Thermoelectrics as materials for sustainable energy technology can offer a considerable benefit in the research of a helpful solution. Particularly, thermoelectric materials have attracted a great deal of attention for their possible applications in heat waste conversion and power generation. Insulating materials and semiconductors have low carrier concentrations and high Seebeck coefficients (S) with high electrical resistivity ρ. An efficient thermoelectric material must provide a delicate balance between the high S, characteristic of an insulator, and the low ρ of metals. Semiconductors are considered the most promising materials having a right balance of these two physical properties. Thanks to the behaviour as semiconductor of some of them, the M–Pn–Ch ternary systems (M = Co, Fe, Ni; Pn = P, As, Sb, Ch = S, Se, Te) are studied both for their interesting structural properties and for their applications as new materials with thermoelectric performances [1–6].
The ternary compound NiSbS exists as a natural mineral named Ullmannite; complete Ni–Sb–S isothermal sections at different temperatures [7–10] and the relative liquidus projection [11] were reported in the literature. Only the NiSbS ternary phase was observed; its structure belongs to the pyrite family [12–14].
The melting temperature of NiSbS was reported in a previous work and corresponds to 781 °C [6]. The Seebeck coefficient (S) and electrical resistivity (ρ) of natural polycrystalline Ullmannite were measured by Johnston et al. [3] as a function of temperature; in particular at room temperature S ~ −9 μV °C−1 and ρ ~ 960 μΩ cm.
The substitution of As for Sb in NiSbS was taken into account in the framework of the investigation for improving the thermoelectric properties in these materials. Different NiAsxSb1−xS quaternary samples were synthesized using increasing quantities of As in the whole compositional range, at selected x values of 0.125, 0.250, 0.333, 0.500, 0.667; a synthetic route similar to that followed for the preparation of the CoSbS and NiSbS phases was adopted [5, 6].
The planning and the synthesis of these complex metallic alloys requires the knowledge of the relative stability of the different phases in the ternary system Ni–As–S also.
Thermodynamics and phase equilibria of the Ni–As–S ternary system were determined [15–17] and reviewed by Tesfaye et al. [11]. Only the ternary NiAsS phase was reported, which exists as natural mineral with the name of Gersdorffite (cP12-P213, NiSbS type). Some magnetic and electrical properties have been reported [16, 18, 19]. Lynch [20] evaluated the standard free energy of formation of NiAsS at temperature between 552 and 702 °C. As no thermoelectric data are reported for this compound, thermal conductivity and thermoelectric measurements will be reported in a future work. Aim of the present work is to report results of the study of thermodynamic and structural properties of NiSbS–As doped phase considered as a possible candidate for thermoelectric applications.
Experimental
A cheap and quick synthetic route was followed to obtain the samples investigated here. Nickel powders (Alfa-Aesar: 99.9 %), antimony rod (Carlo Erba: 99.99 %), arsenic rod (NewMet Koch: 99.999 %) and sulphur powder (Sigma-Aldrich 99.99 mass%) were used as starting materials. Several solid solutions of As–Sb, in different ratios, were obtained starting from small pieces of Sb and As in stoichiometric amounts. They were melted in muffle furnace, in a sealed silica tube under an argon atmosphere at 750 °C for 1 h and then slowly cooled. Appropriate amounts of the so-obtained solid solutions were grounded in agate mortar with the other two elements, Nickel and Sulphur; the powders were then twice pressed (P = 100 MPa) obtaining a pellet. The NiAsxSb1−xS compounds were prepared in muffle furnace by melting the pellet in a silica tube under partial pressure of Ar. The adopted thermal cycle consisted in a slow heating up to 750 °C, keeping at this temperature for 3 h, then at 500 °C for 24 h and finally a slow cooling to room temperature. Microstructural and compositional analyses were performed by scanning electron microscopy (SEM) and energy-dispersive X-ray spectroscopy (EDXS). A scanning electron microscope EVO 40 (Carl Zeiss) was employed, equipped with a Pentafet Link (Oxford Instruments) detector for EDXS analysis. Smooth surfaces for microscopic observation were prepared by standard methods. For the quantitative analysis an acceleration voltage of 20 kV was applied; the corresponding standard used for the calibration was pure cobalt. The X-ray spectra were processed by the software package Inca Energy 4.09 (Oxford Instruments).
X-ray diffraction analysis was used in order to identify the crystal structures of the phases and to determine the lattice parameters. The measurements were performed on powdered samples mounted on a zero-background Si support, by means of a vertical diffractometer (Philips X’pert). X-ray patterns were recorded using the Cu Kα radiation in the 10°–100° 2θ angular range, with a step of 0.03° and a counting time of 3 or 4 s per step.
Differential thermal analysis (DTA) was performed on samples with mass between 150 and 250 mg, in Ta crucibles, sealed under Ar atmosphere, at a scan rate of 5 °C min−1 in a 30 mL min−1 argon flux. The instrument used was a Netzsch 404 C mod. Pegasus, equipped with a temperature controller mod TASC414/c. The measurements were generally executed twice. Onset values were chosen for temperature measurements. No reaction between samples and Ta crucibles was observed.
Structural and microstructural characterisation
The ternary compound NiSbS exists as natural mineral named Ullmannite, and its crystal structure has been known since many years [1, 2]. All compounds within the series NiSb1−xAsxS belong to the same structure type, crystallising in the cubic system with space group P213. The Ullmannite structure belongs to the group of pyrite, from which it can be derived by ordered substitution of Sb and S at the pyrite S site. Indeed, while the Fe position in FeS2 [Wychoff site 4a of space group Pa-3; (0,0,0)] corresponds to that of Ni in NiSbS with only a small variation of the atomic parameter (4a site, x = 0.98), the position of S in FeS2 (8c, x = 0.384) is spit into two independent 4a sites in NiSbS (x Sb = 0.63; x S = 0.38).
This arrangement globally gives rise to a lowering of the symmetry, with the change from the centrosymmetric Pa-3 space group (FeS2) to the acentric P213 group (NiSbS); anyway, it allows a better space filling of the whole structure, as expected considering the different atomic dimensions of Sb and S. This clearly results from the analysis of the bond distances of Sb and S which, even retaining the same tetrahedral coordination adopted by S in FeS2, exhibit significantly different values (Sb–Ni d = 2.563 Å, S–Ni d = 2.376 Å [2]). In Fig. 1 the tridimensional arrangement of the tetrahedra around the sulphur atoms is shown, in a projection along the [100] direction. The Ni atoms at the vertices are shared by three tetrahedra, so that each tetrahedron is connected with nine others. A quite similar connection is observed for the Sb atoms, while Ni atoms have octahedral coordination (3Sb + 3S).
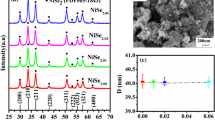
The X-ray powder patterns observed for all NiAs x Sb1−x S samples reveal the presence of only one phase. The analysis of the diffractograms, performed by means of the crystallographic programs Powdercell [21] and Latcon [22], allowed to unambiguously assign the NiSbS type to all studied samples. Very small impurity of As2S3 can sometimes be detected. As a representative example, the experimental pattern of NiSbS is compared with the calculated one in Fig. 2. The very small peaks, not indexed, are due to the presence of impurity of As2S3. The values of lattice parameters are reported in Table 1: very good agreement with Vegard’s law was found, as also shown in the graph of Fig. 3.
The Fig. 4 shows a typical SEM (SE and BSE mode) microphotographs of the examined alloys. All samples with x < 0.66 exhibit a one-phase appearance; the fractures and the holes on the sample surface are probably due to the internal mechanical strains during cooling. The little darker zone on the left is due to the polishing residual. EDXS analyses carried out on all samples show a very good agreement with the nominal compositions. The As-rich domain with x > 0.667 has not been investigated due to the experimental difficulty to obtain a single phase alloy.
Thermal characterisation
The NiSbS melting temperature, previously determined by us using a DSC method [6], was found in agreement with other literature data [4]. Its value is reported in Table 2, together with the melting temperatures measured for the other NiAsxSb1−xS compounds; the corresponding trend as a function of x, plotted in Fig. 5, shows decreasing values up to x ~ 0.25, followed by a regular increase for x > 0.5. This behaviour can be compared with the Sb–As binary phase diagram, redrawn from Okamoto [23] and reported in Fig. 6. The close similarity of the two diagrams is noteworthy, as both present a minimum at nearly the same composition.
As a conclusion we suggest that the quaternary compounds NiAsxSb1−xS could belong with good probability to a pseudo-binary system NiAsS–NiSbS. This is supported by the close Vegard behaviour of the solid solution and by the thermal data, closely related to the binary diagram As–Sb.
Moreover, the difficulty in obtaining single phase alloys in the range of composition 0.66 < x < 1 is probably related to the existence of a wide two phase field in As–Sb phase diagram.
Summary
Different NiAsxSb1−xS samples were synthesized with increasing amounts of As (0 < x < 1), employing a simple synthetic route, using a muffle furnace. Scanning electron microscopy (SEM) equipped with energy-dispersive X-ray spectroscopy (EDXS) was used to investigate the microstructure. X-ray diffraction analysis was employed to identify the crystal structure (cP12-P213 NiSbS type) and to determine the lattice parameters. Good agreement with Vegard’s law was evidenced. Differential thermal analysis (DTA) was performed on all the samples and the melting temperatures were determined. The behaviour of the melting temperatures of the NiAsxSb1−xS compounds was also compared with the Sb–As binary phase diagram and the close analogy of the two diagrams was emphasised.
References
Takèuchi Y. The absolute structure of Ullmannite, NiSbS. Mineral J. 1957;2:90–100.
Foecker AJ, Jeitschko W. The atomic order of the pnictogen and chalcogen atoms in equiatomic ternary compounds TPnCh (T = Ni, Pd; Pn = P, As, Sb; Ch = S, Se, Te). J Solid State Chem. 2001;162:69–78.
Johnston WD, Miller RC, Damon DH. Electrical properties of some compounds having the pyrite or marcasite structure. J Less Common Met. 1965;8:272–87.
Allazov MR, Gulieva ZT. Physicochemical interaction in the CoS–Sb and NiS–Sb systems. Russ J Inorg Chem. 1988;33:1075–8.
Carlini R, Artini C, Borzone G, Masini R, Zanicchi G, Costa GA. Synthesis and characterization of the compound CoSbS. J Therm Anal Calorim. 2011;103:23–7.
Carlini R, Zanicchi G, Borzone G, Parodi N, Costa GA. Synthesis and characterization of the intermetallic compound NiSbS. J Therm Anal Calorim. 2012;108:793–7.
Guertler W, Schach H. Betrachtungen zur theoretischen Metallhüttenkunde. Metall und erz. 1923;20:162–7.
Williams KL, Kullerud G. The Ni–Sb–S system. Carnegie Institute of Washington. Year book. 1970;68:270–3.
Shenck R, Van der Forst P. Gleichgewichtsstudien an erzbildenden sulfiden I. Zeit Anorg Allg Chemie. 1939;241:145–57.
Lange W, Schlegel H. Die Zustandsbilder der Systeme Eisen-Antimon-Schwefel und Kobalt-Antimon-Schwefel. Zeit Metallk. 1951;42:257–68.
Tesfaye Firdu F, Taskinen P. Thermodynamics and phase equilibria in the (Ni, Cu, Zn)–(As, Sb, Bi)–S systems at elevated temperatures (300–900 °C). Espoo: Aalto University Publications in Materials Science and Engineering; 2012. p. 4–58.
Bayliss P. Subdivision of the pyrite group and a chemical and X-ray diffraction investigation of Ullmannite. Can Mineral. 1986;24:27–33.
Bayliss P. Crystal structure refinement of arsenian ullmannite. Am Miner. 1977;62:369–73.
Bayliss P. Isomorphous substitution in synthetic cobaltite and ullmannite. Am Miner. 1969;54:426–30.
Yund RA. The Ni–As–S system. Carnegie. 1959;58:183–4.
Steger JJ, Nahgian H, Arnott RJ, Wold A. Preparation and characterization of the solid solution Co1−x Ni x AsS (x = 1). J Solid State Chem. 1974;11:53–9.
Yund RA. The system Ni–As–S: phase relations and mineralogical significance. Am J Sci. 1962;260:761–82.
Hulliger F. Conductivity behaviour of new compounds with the cobaltite structure. Helvetica Phys Acta. 1962;35:535–7.
Hulliger F. New compounds with the cobaltite structure. Nature. 1963;198:382–3.
Lynch DC. Standard free energy of formation of NiAsS. Metall Trans. 1982;13B:285–8.
Kraus W, Nolze G. PowderCell-A program for the representation and manipulation of crystal structure and calculation of the resulting X-ray powder patterns. J Appl Crystallogr. 1996;29:301–3.
Schwarzenbach D. Latcon: refine lattice parameters. Lausanne: University of Lausanne; 1966.
Okamoto H, Desk Handbook: Phase Diagrams for Binary Alloys. Materials Park: ASM International; 2000. p. 63.
Goncharov EG, Cherpakova GV, Pshestanchik VR. Thermodynamic assesment of interaction in the arsenic-antimony system, Zh Fiz Khim. 1977;51:2401 (in Russian, Trans., Russ J Phys Chem. 1977;51:1411).
Ugai YA, Samoilov AM, Semenova GV, Goncharov EG. Zh Fiz Khim. 1986;60:25–28 (in Russian, Trans., Russ J Phys Chem. 1977;60:14–16).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Carlini, R., Macciò, D., Pani, M. et al. Synthesis and thermal properties of NiSbS–As doped phase. J Therm Anal Calorim 112, 513–517 (2013). https://doi.org/10.1007/s10973-012-2841-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-012-2841-1