Abstract
New poly(azo) amino-chitosan compounds were obtained from the azo coupling reaction of N-benzyl chitosan and diazonium salts. The thermal behavior of these compounds was studied by thermogravimetric analysis (TG), differential thermogravimetric analysis (DTG), TG coupled with a Fourier-transform infrared, and differential scanning calorimetry (DSC). TG/DTG curves of chitin–chitosan polymer showed two thermal events attributed to water loss and decomposition of the polysaccharide after cross-linking reactions. Thermal analysis of the poly(azo) amino-chitosan compounds showed that the decomposition temperatures decreased when compared to the starting chitin–chitosan and N-benzyl chitosan. DSC results showed an agreement with the TG/DTG analyses. Thermal behavior of poly(azo) amino-chitosans suggest that these compounds could be considered as potential thermal sensors.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chitosan is a natural linear copolymer consisting of β-(1 → 4)-linked 2-acetamido-2-deoxy-β-d-glucopyranose and 2-amino-2-deoxy-β-d-glucopyranose. It is obtained by the deacetylation of chitin, a structural polysaccharide which is present in the shells of crustaceans and insects [1].
In view of the large number of polar groups present in the polymer chain, chitosan is hydrophilic and has a high affinity for water molecules. The solubility of chitosan depends on the distribution of free amino and N-acetyl groups and its molecular weight; therefore, only aqueous acid solutions are used for solubilization of chitosan. Due to its properties such as biocompatibility, biodegradability, and no toxicity, it has been widely used in fields like food, cosmetics, biomedicine [2], agriculture [3], and wastewater treatment [4].
The reactive hydroxyl (C-3 and C-6) and amino groups (C-2) are subject to chemical transformations and can modify some mechanical and physical properties of chitosan.
The presence of free nucleophilic amino groups in the macromolecule chain allows the preparation of N-alkyl chitosan derivatives via reductive amination in which the chitosan amino groups react with aldehydes to form a Schiff’s bases that are later treated with a reducing agent to yield the corresponding secondary amine compounds.
Azo dyes contain groups (–N=N–) linked to aromatic sp 2-hybridized carbon atoms and provide a great variety of applications such as colorants for textile industries, drugs, digital printing and photography [5], and food and cosmetic. Some dyes can also be used in the biomedical field [6], optical storage media [7], and electronic devices [8].
The formation of poly(azo) amino-chitosan compounds occurs at low temperatures in the presence of aromatic diazonium salts and adequate solvent.
Among several techniques, thermal analysis has been largely employed for the characterization of chitosan materials. Some results of thermal analysis of these materials have been reported in the literature, such as water content [9], thermal decomposition, and stability [10]. The present work describes the thermal study of new poly(azo) amino-chitosan compounds synthesized by reductive amination and subsequent azo coupling reaction. Part of the results described in this work has already been presented in the poster session of VIII Congresso Brasileiro de Análise Térmica e Calorimetria. III Congresso Pan-Americano de Análise Térmica e Calorimetria [11].
Experimental
Reagents
Chitosan with molecular weight of 75–160 kDa was purchased from Sigma-Aldrich and used without further purification. Its degree of deacetylation was 60 % and was determined by FT-IR analysis [12]. The other reagents were of analytical grade and also used without further purification.
General procedure for synthesis of N-benzyl chitosan
The N-alkylation of chitosan was made following the procedure previously reported in the literature [13]. N-benzyl chitosan was prepared by dissolving 0.5 g of chitosan in 1 % aqueous acetic acid (pH 4). After complete dissolution, an ethanolic solution of benzaldehyde (2 mmol, 0.21 g) was added and resulting mixture was stirred at 60 °C for 18 h yielding a yellow gel. Then, 2 mmol (0.12 g) of NABH3CN was added to the yellow gel and the mixture was stirred at room temperature for 24 h. Afterward the solvent was separated using a rotary evaporator under reduced pressure and the product was washed with water and ethanol. The white solid was dried for 1 h at 60 °C.
General procedure for synthesis of poly(azo) amino-chitosan compounds
2 mmol of 4-chloro or 4-nitro-benzenediazoniumtetrafluoroborate were dissolved in a minimal volume of DMF and 0.5 g of N-benzyl chitosan was slowly added to the solution. The mixture was stirred for 20 min at 0–5 °C. Finally, the products were filtered and washed with ethanol. The products poly-(4-(4-chlorophenyl)diazenyl)-N-benzyl chitosan and poly-(4-(4-nitrophenyl)diazenyl)-N-benzyl chitosan were obtained as yellow and red gels, respectively.
Proton nuclear magnetic resonance (1H NMR)
1H NMR measurements were performed on an AVANCE III NMR spectrometer at 400 MHz at 25 °C. The spectra were acquired by dissolving chitin–chitosan polymer and N-benzyl chitosan compound in D2O/HCl (0.1 mol L−1). The poly(azo) amino-chitosan compounds were dissolved in DMSO-d6.
Thermogravimetric analysis (TG)
The study was carried out using a coupled TG/FT-IR system. TG measurements were carried out with a Netzsch TG 209. The powdered samples (~5 mg) were placed into aluminum pans and heated at 10° min−1 under a dynamic nitrogen atmosphere at a flow rate of 25 mL min−1 from room temperature to 700 °C. The FT-IR spectra were recorded in a range of 400–4,000 cm−1 with 4 cm−1 spectral resolution and 32 scans. The gas products evaporated in the TG are channeled into a chamber with ZnSe windows. The infrared beam passes through the chamber, interacts with the gases, and reaches a detector type mercury(II)cadmium(II)telluride (MCT).
Differential scanning calorimetry (DSC)
DSC measurements were performed in a Netzsch DSC 204 using sample mass of 5 mg in a covered aluminum pan. An empty pan was used as reference. The samples were heated at 10° min−1 from room temperature to 500 °C with a gas flow rate of 15 mL min−1.
Results and discussion
N-benzyl chitosan was conveniently achieved by reacting the biopolymer with benzaldehyde in the presence of a reducing agent. Figure 1 shows the reaction for the synthesis of N-benzyl chitosan.
The azo coupling reactions occur via electrophilic aromatic substitution of the benzene derivative by electrophilic aromatic diazonium compounds [14]. The insertion of new hydrophobic groups in the polymer side chain enhanced the solubility of the compounds due to the decrease in crystallinity.
Figure 2 shows the reaction for the synthesis of poly(azo) amino-chitosan compounds.
1H NMR measurements
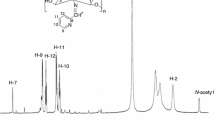
Figure 3 shows the 1H NMR spectrum of chitin–chitosan polymer and its derivatives. The chitin–chitosan spectrum presents the signal at 1.91 ppm assigned to the N-acetyl protons of GlcNAc (–COCH3), at 3.08 ppm attributed to the proton in position 2 (H2) of GlcN residue and the proton (H2′) of the acetamide groups of GlcNAc that resonates at 3.47 ppm. The signals observed in the region of 3.64–3.80 ppm are attributed to the protons in positions H3, H4, H5, and H6 of the glucosamine ring. The signal at 4.48 ppm refers to the proton (H1′), bonded to carbon C4. In the region of 4.70 ppm, it can be observed that the anomeric proton signal (H1) of glucosamine ring superposed to the solvent signal (HDO) [15]. The 1H NMR spectrum of N-benzyl chitosan, Fig. 3, presents new signals which confirmed the modification in the polymeric chain. The spectrum exhibits signals in the region of 7.31 and 7.69 ppm, which are attributed to the protons of the aromatic ring (a,b,c) bonded to the polymer structure. The signals between 4.39 and 4.46 ppm correspond to the methylene protons (H8), to H1′, and to the anomeric proton (H1). The signal at 1.85 ppm is assigned to methyl protons of acetyl units and the protons H2, H2′, H3, H4, H5, and H6 resonate in the region of 3.64 and 3.86 ppm. The 1H NMR spectra of the poly(azo) amino-chitosan compounds are also shown in Fig. 3. An increase of signals can be observed in the region of 7.30 and 8.30 (a,b,d,e), which suggests the presence of another aromatic ring bonded to the modified polymeric chain and confirms the synthesis of the azo compounds.
Thermogravimetric analysis (TG) and TG/FT-IR of gas products
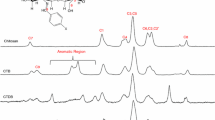
The thermal stability of chitosan and chitosan derivatives were studied by thermogravimetric analysis. Figure 4a shows the TG and DTG curves of chitosan. The first thermal event is observed at 62 °C with a weight loss of 11 %, which is assigned to the loss of water bounded to the polymeric structure by hydroxyl and amine groups. From TG, loss of water was observed to be occurring at a temperature below 100 °C, according to literature it means that this water could be physically adsorbed and/or weakly hydrogen-bonded to chitin–chitosan molecules [16]. The second thermal event reaches a maximum at 277 °C with a weight loss of 46 %. It corresponds to the decomposition of chitin–chitosan polymer [17]. Also a weight loss of 18 % and a residual mass of 25 % was observed, showing that not all the chitosan was decomposed until 700 °C.
In agreement to the literature [18], it is reported that at this temperature the thermal degradation of the pyranose ring is observed, with the rupture of the glycosidic linkages between the glucosamine and N-acetylglucosamine rings. Pawlak and Mucha [19] have confirmed by thermogravimetric and FT-IR studies that the second decomposition step may be due to the thermal degradation of a new cross-linking formed by the thermal decomposition of functional amino group.
Apparently, N-benzyl chitosan, Fig. 4b showed a slightly higher water holding capacity than the chitin–chitosan polymer due to the decrease of chitosan crystallinity created by the N-benzylation along the polymer chain [20].
In order to investigate the thermal degradation process, FT-IR spectra was recorded at the temperature values in which mass losses were observed in the TG/DTG curves. Figure 5a shows the FT-IR bands of chitin–chitosan degradation recorded at 62 and 277 °C. At 62 °C the bands at 3,268 cm−1 assigned to the axial stretching of O–H and N–H bonds are observed and at 2,357 cm−1 bands corresponding to CO2 due to the oxidation of aldehyde fragments resulting from thermal degradation of the polymer chain are observed. The loss of amino groups, probably as ammonia or a volatile amine, by the band at 1,554 cm−1, and acetamide groups which correspond to the bands at 1,694 and 1,644 cm−1, attributed to amide I and amide II vibrations can also be seen. The bands at 1,427 and 1,383 cm−1 are assigned to C–H angular deformation and C–N axial stretching, respectively. The FT-IR spectrum of the polymer at 277 °C shows an increase in intensity of the band at 1,747 cm−1 which corresponds to the aldehyde carbonyl group which is formed as a product of the polymer degradation [21, 22]. The band at 1,180 cm−1 which is assigned to C–O–C stretching of glycosidic bond is also observed.
The FT-IR spectrum of N-benzyl chitosan at 62 °C, Fig. 5b, shows the beginning of the compound degradation and an intense band at 3,269 cm−1 attributed to O–H stretching which confirms the loss of water. At 262 °C, an increase in intensity of the bands at 2358, 1735, 1646, 1530, 1462, and 1394 cm−1 due to the polymer degradation is observed. At 404 °C, the main bands observed are at 3,269 cm−1 attributed to O–H stretching, at 2,358 cm−1 due to the loss of CO2, at 1,735 cm−1 assigned to the aldehyde carbonyl group [21, 22], and at 1,530 cm−1 which corresponds to the N–H angular deformation.
Figure 6a shows the TG/DTG curves of poly-(4-(4-chlorophenyl)diazenyl)-N-benzyl chitosan. The first thermal event is observed at 112 °C with 79 % weight loss. It is attributed to dehydration, cleavage of azo linkage, loss of the benzene rings bounded to the polymer backbone, and a possible depolymerization of chitin–chitosan. The residual decomposition reactions can be seen at 195 °C with weight loss of 12 %. A residual mass of 9 % was observed for this compound.
The TG/DTG curves of poly-(4-(4-nitrophenyl)diazenyl)-N-benzyl chitosan have similar thermal behavior of poly-N-4-(4′-nitrophenylazo)benzyl chitosan. The thermal decomposition step appears at 112 °C with weight loss of 81 %, and is attributed to water desorption from polymer chain. The polymer degradation is seen at 172 °C with weight loss of 8 %. The residual mass is 11 %. The thermal events observed for this compound are shown in Fig. 6b.
Figure 7a shows the FT-IR spectrum of poly-(4-(4-chlorophenyl)diazenyl)-N-benzyl chitosan. At 112 °C, the main bands observed are at 2,939 cm−1 attributed to the axial stretching of C–H bonds, at 1,713 cm−1 assigned to the carbonyl group formed during thermal degradation [21, 22], at 1,504 cm−1 due to N–H angular deformation, and the bands at 1,381 and 1,080 cm−1 which are attributed to C–N axial stretching and C–O–C stretching of glycosidic bond, respectively. At 195 °C, an increase in intensity of the bands due to the thermal degradation of the compound is observed. In the spectra the appearance of new bands at 1,460 cm−1 assigned to N=N stretching of azo groups and at 1,421 cm−1 which corresponds to the deformation of C=C bonds of aromatic rings present in the compound can also be seen.
The FT-IR spectrum of poly-(4-(4-nitrophenyl)diazenyl)-N-benzyl chitosan is shown in Fig. 7b. At 112 °C, the bands at 2,930 cm−1 corresponding to the axial stretching of C–H bonds, at 1,719 cm−1 due to ring opening and formation of aldehyde groups [21, 22], and the bands at 1,388 and 1,080 cm−1 which are attributed to C–N axial stretching and C–O–C stretching of glycosidic bond, respectively, can be seen. At 172 °C, the main bands observed are at 2930, 1718, 1388, and 1080 cm−1. An increase in intensity of the bands at 2,363 cm−1 attributed to the loss of CO2 and at 1,547 cm−1 which corresponds to N–H angular deformation of deacetylated units can also be observed. Besides, it is possible to observe in the spectrum, at this temperature, the band which corresponds to the stretching of azo groups (N=N) at 1,496 cm−1.
Differential scanning calorimetry (DSC)
Figure 8a shows the DSC curve of chitin–chitosan. The endothermic peak is observed at 95 °C and is attributed to the loss of water content in the polymer. The exothermic peak that appears at 306 °C corresponds to the decomposition of the polymer chain [23].
A similar thermal transition behavior was observed in the N-benzyl chitosan derivative, Fig. 8b, in which an endothermic peak at 104 °C related to the evaporation of water present in the sample is observed. The exothermic peak at 276 °C is associated with polymer decomposition.
The DSC curves of N-benzyl chitosan showed that the derivative exhibited stronger interactions with water molecules and it is less stable than chitin–chitosan polymer due to its lower decomposition temperature.
The DSC curve of poly-(4-(4-chlorophenyl)diazenyl)-N-benzyl chitosan is shown in Fig. 9a. It exhibits two endothermic peaks which correspond to the thermal effects observed in TG/DTG curves. These endothermic peaks show the maximum at 116 °C and a shoulder at 155 °C and are associated with loss of water molecules content in the compound and rupture of the new substituents bounded to the polymer chain. The exothermic peak at 216 °C corresponds to the chitin–chitosan thermal degradation.
Similarly, the DSC curves of poly-(4-(4-nitrophenyl)diazenyl)-N-benzyl chitosan are shown in Fig. 9b. It exhibits two wide endothermic peaks at 125 and 158 °C. The exothermic peak, which appears at 304 °C, corresponds to the decomposition of chitin–chitosan backbone.
Conclusions
N-benzyl chitosan and poly(azo) amino-chitosan compounds were successfully synthesized by reductive amination and azo coupling reactions, respectively. TG/DTG measurements show a decrease in the decomposition temperature of chitosan derivatives when compared to non-modified polymer. The poly(azo) amino-chitosan compounds cannot withstand temperatures higher than 172 and 195 °C. The thermograms suggest that water molecules could be physically adsorbed and/or weakly hydrogen-bonded to the chitin–chitosan chain because the range of temperature corresponding to dehydration is observed below 100 °C. DSC curves confirm the results observed for TG/DTG measurements. Tg is not observed for the studied molecules under the experimental conditions of measurements.
References
Kurita K. Chitin and chitosan: functional biopolymers from marine crustaceans. Marine Biotechnol. 2006;8:203–26.
Riva R, Ragelle H, des Rieux A, Duhem N, Jerome C, Preat V. Chitosan and chitosan derivatives in drug delivery and tissue engineering. In: Jayakumar RPMMRAA, editor. Chitosan for biomaterials II. Advances in polymer science, 2011. p. 19–44.
Devlieghere F, Vermeulen A, Debevere J. Chitosan: antimicrobial activity, interactions with food components and applicability as a coating on fruit and vegetables. Food Microbiol. 2004;21:703–14.
Hussein MHM, El-Hady MF, Sayed WM, Hefni H. Preparation of some chitosan heavy metal complexes and study of its properties. Polym Sci Ser A. 2012;54:113–24.
Landl M, Šimon P, Breza M. Synthesis and spectra of tris(4-dimethylaminophenyl)divinylenes. Dyes Pigm. 1999;40:43–51.
Gopalakrishnan S, Nevaditha NT, Mythili CV. Antibacterial activity of azo compounds synthesized from the natural renewable source, cardanol. J Chem Pharm Res. 2011;3:490–7.
Manickasundaram S, Kannan P, Hassan QMA, Palanisamy PK. Azo dye based poly(alkyloxymethacrylate)s and their spacer effect on optical data storage. J Mater Sci. 2008;19:1045–53.
Hong Y-G, Gu J-D. Physiology and biochemistry of reduction of azo compounds by Shewanella strains relevant to electron transport chain. Appl Microbiol Biotechnol. 2010;88:637–43.
Khalid MN, Agnely F, Yagoubi N, Grossiord JL, Couarraze G. Water state characterization, swelling behavior, thermal and mechanical properties of chitosan based networks. Eur J Pharm Sci. 2002;15:425–32.
Cardenas G, Bernal L, Tagle LH. Thermogravimetric studies of chitosan derivatives. Thermochim Acta. 1992;195:33–8.
VIII Congresso Brasileiro de Análise Térmica e Calorimetria. III Congresso Pan-Americano de Análise Térmica e Calorimetria. 01–04 April, 2012, Campos do Jordão, São Paulo, Brazil.
Brugnerotto J, Lizardi J, Goycoolea FM, Arguelles-Monal W, Desbrieres J, Rinaudo M. An infrared investigation in relation with chitin and chitosan characterization. Polymer. 2001;42:3569–80.
Borch RF, Bernstei Md, Durst HD. Cyanohydridoborate anion as a selective reducing agent. J Am Chem Soc. 1971;93:2897–904.
Langhals H. Color chemistry. Synthesis, properties and applications of organic dyes and pigments, 3rd revised edition. Heinrich Zollinger. Angewandte Chemie International Edition. 2004;43:5291–2.
Kim S. Chitin, chitosan, oligosaccharides and their derivatives, biological activities and applications. USA: CRC Press; 2011. p. 149–66.
Zawadzki J, Kaczmarek H. Thermal treatment of chitosan in various conditions. Carbohydr Polym. 2010;80:394–400.
López FA, Mercê ALR, Alguacil FJ, López-Delgado A. A kinetic study on the thermal behaviour of chitosan. J Therm Anal Calorim. 2008;91:633–9.
Tang WJ, Wang CX, Donghua C. Kinetic studies on the pyrolysis of chitin and chitosan. Polym Degrad Stab. 2005;87:389–94.
Pawlak A, Mucha M. Thermogravimetric and FTIR studies of chitosan blends. Thermochim Acta. 2003;409:95–7.
Sajomsang W, Tantayanon S, Tangpasuthadol V, Thatte M, William H, Daly HW. Synthesis and characterization of N-aryl chitosan derivatives. Int J Biol Macromol. 2008;43:79–87.
Zeng L, Qin C, Wang L, Li W. Volatile compounds formed from the pyrolysis of chitosan. Carbohydr Polym. 2011;83:1553–7.
Koll P, Borchers G, Metzger JO. Thermal degradation of chitin and cellulose. J Anal Appl Pyrol. 1991;19:119–29.
Guinesi LS, Cavalheiro ETG. The use of DSC curves to determine the acetylation degree of chitin/chitosan samples. Thermochim Acta. 2006;444:128–33.
Acknowledgements
The authors gratefully thank Fundação de Apoio a Pesquisa do Estado de São Paulo (FAPESP), Programa de Pós-graduação em Ciência e Tecnologia de Materiais (POSMAT), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for financial support and post-graduation fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pereira, F.S., da Silva Agostini, D.L., Job, A.E. et al. Thermal studies of chitin–chitosan derivatives. J Therm Anal Calorim 114, 321–327 (2013). https://doi.org/10.1007/s10973-012-2835-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-012-2835-z