Abstract
The restriction to the use of synthetic antioxidants has fostered the research on natural antioxidants, taking into account that the prolonged usage of these substances can harm seriously the human being provoking degenerative diseases. In the present study, the antioxidant effect of the ethanolic rosemary (Rosmarinus officinalis L.) extract on the oxidative stability of edible vegetable oils was investigated by means of the pressurized differential scanning calorimetry (PDSC) and oven test techniques. The rosemary extract, at the concentration of 2,000 mg kg−1, as well as the synthetic antioxidant tert-butylhydroquinone (TBHQ) at the concentrations of 100 and 200 mg kg−1 were added to samples of sunflower oil, corn oil, and soybean oil. The fatty acid profiles of the vegetable oils were determined by gas chromatography–mass spectrometry confirming the elevated contents of unsaturated fatty acids. The thermogravimetric analysis showed that the rosemary extract is stable at the frying temperature of the oils. The results of the oxidative stability demonstrated that the extract of Rosmarinus officinalis displayed a more effective protective action in the PDSC technique, when compared with the synthetic antioxidant TBHQ, indicating that it is a promising source of natural antioxidants for edible vegetable oils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Edible oils contain polyunsaturated fatty acids, which are important substances on the biological and nutritional points of view. In relation to the chemical structure, fatty acids present double bonds that make them prone to oxidative reactions. Such reactions may compromise their stability, causing modifications in the organoleptic, nutritional and functional properties, besides giving rise to chemical compounds which are harmful to the human health [1].
The lipid oxidation is commonly controlled by the addition of antioxidants [1, 2]. The synthetic antioxidants butylated hydroxyanisole (BHA), tert-butylhydroquinone (TBHQ), propyl gallate (PG), 2,4,5-trihydroxybutyrophenone (THBP), di-tert butyl-4-hydroxymethylphenol (IONOX-100) are widely employed by the industry [2, 3] due to their low cost and good performance. However, the prolonged usage of these substances can cause serious harms to the human being, provoking degenerative diseases [4]. Thus, natural sources of antioxidants [5] have been a feasible alternative to face the inconveniences caused by synthetic antioxidants.
Among the diverse classes of antioxidant substances of natural occurrence, the phenolic compounds have received much attention in the last years, chiefly as they inhibit in vivo and in vitro lipid oxidation [2, 6].
Foods such as fruits, herbs, and cereals are among the sources of natural antioxidants that have been quite utilized [7, 8]. In relation to herbs, the extract of rosemary (Rosmarinus officinalis L.) has pointed out as it displays an elevated antioxidant power and provides an excellent protection factor in the oxidative stability of lipid matrices [9]. This activity is associated to the presence of phenolic acids, flavonoids, diterpenoids, and phenolic triterpenes [10].
Therefore, the present study had an objective to evaluate the antioxidant efficiency of rosemary extract, in vegetable oils, by means of accelerated methods of assessing the oxidative stability, namely PDSC and oven test.
Experimental
Materials
Refined vegetable oils (sunflower, corn, and soybean) and dried rosemary leaves were acquired from the local market. According to the manufacturer specifications, the vegetable oil samples were free from antioxidants. The synthetic antioxidant TBHQ (tert-butylhydroquinone) was supplied by Sigma Aldrich and the remaining reagents and solvents, of analytical degree, by FMAIA.
Methods
Preparation of the antioxidant extracts
The rosemary extract was obtained from 170.3 g of dried and crushed leaves, submerged in 960 mL of ethanol for a period of 15 days at room temperature. A filtration was carried to separate the plant residue and then the extract was concentrated by rotoevaporation at temperature 78 °C, obtaining 27.8 g of dry extract, corresponding to a 16.3 % yield. The extract was stored in a glass container, protected from light and room temperature up to the moment of being utilized.
Thermal analyses
The thermogravimetric curves (TG/DTA) of the rosemary extract were obtained in a model DTG-60H simultaneous thermal analyzer from Shimadzu. The tests non-isothermic were carried out using about 10 mg samples in an alumina pan and synthetic air atmosphere, with a flow rate of 50 mL min−1, heating rate of 10 °C min−1, and temperature range of 25–1,000 °C. The isothermal curves were obtained in a synthetic air atmosphere, using approximately 10 mg of sample in an alumina pan, with a flow rate of 50 mL min−1. The following heating schedule was used: an initial heating rate of 10 °C min−1 was maintained until the temperature reached 100 °C, followed by a rate of 2 °C min−1 until reaching 110 °C, at which point the isotherm measurement was performed.
Determination of the fatty acid profiles of the vegetable oils
The vegetable oils were transesterified according to the IUPAC standard method 2.301 [11]. The esters were analyzed by a Gas Chromatography–Mass Spectrometry (GC–MS) apparatus model GCMS-QP2010 from Shimadzu, equipped with a split injector.
Preparation of the oil samples, with and without additives
The rosemary extract at the concentration of 2,000 mg kg−1, and also the synthetic antioxidant TBHQ at the concentrations of 100 and 200 mg kg−1, were added to the samples of sunflower, corn, and soybean oils. Table 1 shows the codes used for the samples of vegetable oils, with and without additives.
Pressurized differential scanning calorimetry
The PDSC curves of the samples were obtained by means of a pressurized differential scanning calorimeter, PDSC, model DSC Q1000 equipment from TA Instruments, coupled to a pressure cell. The tests were performed in the isothermal mode (110 °C) utilizing an aluminum pan of approximately 10 mg sample, under an oxygen atmosphere with initial pressure of 203 psi (roughly 1,400 kPa) and heating rate of 10 °C min−1.
Oven test
The accelerated tests in an oven with air circulation were conducted for 29 days at the temperature of 60 ± 5 °C. A volume of 35 mL of each sample was placed in a glass container. The containers were maintained within the oven to allow the direct contact with air. At intervals of 7 days, aliquots from the samples were taken and were analyzed to perform the following physico-chemical tests: acid value, peroxide value, conjugated dienes, according to the methods NBR 11115 ABNT [12], Cd 8-53 AOCS, and Ti 1a-64 AOCS [13], respectively. The absorbance was measured using a model UV-2550 UV–Vis Shimadzu spectrophotometer at 232 nm against a blank of isooctane.
Results and discussion
Fatty acid profile of the vegetable oils
Table 2 presents the fatty acid profile of the vegetable oils investigated.
According to the data from Table 2, it is noticed that the vegetable oils studied presented elevated amounts of unsaturated fatty acids, what agrees with the data reported in the literature [14–16].
The polyunsaturated fatty acids act in several physiological and metabolic processes. These acids are not synthesized by the human organism, thus they ought to be supplied by the food diet, for a good maintenance of health. Linoleic (18:2 ω-6) and α-linolenic (C18:3 ω-3) acids are included in this group. These two fatty acids are precursors of the polyunsaturated fatty acids of long chains from the series ω-3 and ω-6, like arachidonic acid (C20:4 ω-6) and docosahexaenoic acid (22:6 ω-3) [1].
Sunflower oil displayed the highest content of unsaturated fatty acids, 89.5 %, corresponding to 32.6 % of monounsaturated fatty acids (MUFA) and 56.9 % of polyunsaturated fatty acids (PUFA). Corn oil also showed a high content of unsaturated fatty acids, 84.5 %, displaying amounts of MUFA higher than the remaining oils and soybean oil was the one that showed the smallest amount of unsaturated fatty acids, 76.3 %.
Aued-Pimentel et al. (2009) [17], studying refined vegetable oils, found smaller contents of oleic acid (34.44–35.68 %) and linoleic acid (22.57–25.82 %) in corn oil and sunflower oil, respectively. As for the soybean oil, the amounts found for oleic acid (22.57–25.82 %) and linoleic acid (48.11–54.65 %) were similar to the values obtained in the present study.
Oxidative stability
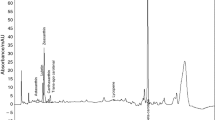
The values of oxidative induction time (OIT), Table 3, obtained by analyzing the PDSC curves, Fig. 1, showed that corn oil is 2.6 times more stable than sunflower oil and 1.1 times more stable than soybean oil.
These results are within the expected range, as although sunflower oil did not show detectable amounts of linolenic acid, it was the vegetable oil with the highest percentage of linoleic acid. Such higher tendency to oxidation of sunflower oil was also noticed upon the application of Eq. 1. Such equation was formulated based on kinetic studies and it estimates the oxidability of a vegetable oil, from the composition of its polyunsaturated fatty acids [18]. Upon the usage of Eq. 1, the oxidability values were calculated as: sunflower oil (0.57), soybean oil (0.55), and corn oil (0.45).
-
In which OX = oxidability of the vegetable oil/%
-
% O = content of oleic acid/mass %
-
% L = content of linoleic acid/mass %
-
% Ln = content of linolenic acid/mass %
In the evaluation of the oxidative stability of the vegetable oils with the addition of additives, it was noticed that the antioxidant TBHQ showed a small antioxidant effect in the sunflower and soybean oils, in the two concentrations investigated. In relation to corn oil, it displayed a pro-oxidant action, reducing the OIT values in the range from 34.2 to 33.1 %, as compared TCO100 and TCO200 samples, respectively. It should be stressed that 200 mg kg−1 is the highest concentration of TBHQ allowed by the Brazilian legislation [19]. This result, however, does not indicate that TBHQ is an antioxidant that cannot be applied to corn oil; it only indicates that the experimental conditions of pressure and temperature, in the present study, were not adequate for the evaluation of the antioxidant efficiency of TBHQ to corn oil.
The oxidative stability of the vegetable oils with the addition of rosemary extract, at the concentration of 2,000 mg kg−1 was also assessed. Differently to the synthetic antioxidant TBHQ, the rosemary extract showed a remarkable antioxidant action in the three vegetable oils, moreover for the case of corn oil. This promising effect of rosemary was already verified in lipid systems, using a chloroform extract of Rosmarinus officinalis in canola oil, by means of the accelerated methods Oxidrograph and Rancimat [20].
Thermogravimetric studies have shown that the stability of several antioxidants decrease with the increase of temperature [21]. The TG/DTG curves of the rosemary extract obtained in the present study (Fig. 2a) showed that it may be a thermostable antioxidant, as it showed a mass loss of only 6 % up to 190 °C (Table 4). Such loss is probably ascribed to the presence of moisture, traces of ethanol––the solvent used to obtain the extract and volatile phenolic compounds.
The second step, from 190 to 462 °C, presented a mass loss of 88.6 %. This loss may also be attributed to the volatilization/decomposition of bioactive constituents, possibly from phenolic diterpenes, such as carnosic acid and carnosol, as well as from the rosmarinic acid.
Finally the third step, in the range from 462 to 1,000 °C, with a mass loss of 5.8 %, can be related to inorganic compounds.
In the isothermal mode at 110 °C (Fig. 2b), the time required to the rosemary extract to lose 6 % mass was of 76 min, and the remaining mass was kept stable for 9 h. This evaluation corroborated the results obtained by PDSC, showing that at the temperature of the tests, the rosemary extract remained stable and active.
Accelerated oven test
The samples submitted to aging in an oven were evaluated according to the tests: acid value, peroxide value, and conjugated dienes.
The acid values for the oil samples with and without additives varied from 0.06 to 0.4 mg KOH kg−1. No meaningful changes were noticed in this parameter during the period of evaluation for all the samples, which kept their acid values below the limit established by ANVISA of 0.6 mg KOH kg−1 [22].
Figure 3 shows the increase of the peroxide values of the samples during storage. Peroxides and hydroperoxides are products that represent the beginning of the lipoxidation [1], a step in which the chain-breaker antioxidants can interrupt the process and restore the fatty acid chain.
Conversely to the acid values, the peroxide values increased during the storage. For the vegetable oils without additives, the peroxide values (PV) were no bigger than the maximum limit established by ANVISA, of 10 meq kg−1 [22].
It can be noticed that the samples of soybean oil presented a small variation among the peroxide values until the 14 day of storage. Figure 3c shows that, at the end of storage period, the rosemary extract increased the protection at 44 %, when compared to the soybean oil without additives. Besides, it is observed that the rosemary extract, for the corn oil (Fig. 3b), acted as a good antioxidant, protecting the oil with a protection factor of 60 %. The protection factor was also verified for the antioxidant TBHQ at the concentration of 100 mg kg−1. In the samples of sunflower oil, both rosemary extract and TBHQ were efficient in controlling the formation of peroxide; however, the effect of both antioxidants was less effective than other two vegetable oils (Fig. 3a).
Several studies have shown that TBHQ is efficient in delaying the oxidative processes in lipid matrices, when added at the maximum amount allowed of 200 mg kg−1 [23, 24]. According to the results reported by Almeida-Doria et al. (2000), TBHQ added to soybean oil obtained a better result against the peroxide than the ethanolic extracts of rosemary and marjoram, during storage at 63 °C [25]. Nevertheless, the results utilizing the rosemary extract were shown promising, chiefly when utilized with corn oil. It is important to stress that the rosemary extract is a source of a natural antioxidant, whereas TBHQ is a synthetic antioxidant, of questionable use in several countries, once its prolonged usage is harmful to health [4].
The presence of conjugated dienes (CD), similarly to the peroxide value, is a parameter for the determination of the oxidative stability of vegetable oils. It is evaluated by the absorbance at the wavelength of 232 nm. The formation of hydroperoxides coincides with the conjugation of the double bonds in polyunsaturated fatty acids (PUFA) [26].
The CD values of the samples during storage are displayed in Fig. 4.
It is observed that a gradual increase occurred in the formation of conjugated dienes, in all the samples. Up to the 14 day, the samples of sunflower oil, corn oil, and soybean oil, with the addition of rosemary extract and TBHQ showed low values of CD, differently to the other samples. This occurs probably by the protecting effect of the antioxidants in inhibiting the formation of the primary oxidation products. After the 21st day, a rapid rise in the CD values was observed for the samples without additives.
The CD values for the samples without additives, conversely to what was observed for the peroxides, revealed a protection factor effect similar among the antioxidants, showing that the activity of an antioxidant cannot be determined by only one parameter.
Conclusions
The results obtained in the present study showed that the rosemary extract is a promising source of natural antioxidants for vegetable oils, being able to protect the oils against oxidative processes, even at high temperatures, once its efficiency measured by the PDSC technique was better than the one of the synthetic antioxidant TBHQ. Another parameter to be taken into account deals with the thermal stability of the rosemary extract, which, by means of thermogravimetric analyses was shown to be stable at the frying temperature of the oils, a property that is not found in the majority of the antioxidants commonly used in edible oils.
References
Shahidi F, Zhong Y. Lipid oxidation and improving the oxidative stability. Chem Soc Rev. 2010;39:4067–79.
Shahidi F. Antioxidants in food and food antioxidants. Nahrung. 2000;44:158–63.
Guo L, Xie MY, Yan AP, Wan YQ, Wu YM. Simultaneous determination of five synthetic antioxidants in edible vegetable oil by GC–MS. Anal Bioanal Chem. 2006;386:1881–7.
Ito N, Hirose M, Fukushima S, Tsuda H, Shirai T, Tatematsu M. Studies on antioxidants: Their carcinogenic and modifying effects on chemical carcinogenesis. Food Chem Toxicol. 1986;24:1071–82.
Ramalho VC, Jorge N. Antioxidants used in oils, fats and fatty foods. Quim Nova. 2006;29:755–60.
Soares SE. Ácidos fenólicos como antioxidantes. Rev Nutr. 2002;15:71–81.
Yanishlieva NV, Marinova E, Pokorný J. Natural antioxidants from herbs and spices. Eur J Lipid Sci Tech. 2006;108:776–93.
Manach C, Scalbert A, Morand C, Rémésy C, Jiménez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr. 2004;79:727–47.
Carvalho RN, Moura LS, Rosa PTV, Meireles MAA. Supercritical fluid extraction from rosemary (Rosmarinus officinalis): kinetic data, extract’s global yield, composition, and antioxidant activity. J Supercrit Fluid. 2005;35:197–204.
Mahmoud AA, Al-Shihry SS, Son BW. Diterpenoid quinones from (Rosmarinus officinalis L.). Phytochemistry. 2005;66:1685–90.
International Union of Pure and Applied Chemistry (IUPAC). Standard methods for the analysis of oils, fats and derivatives. 7th ed. Boston: Blackwell Scientific Publications; 1987.
Associação Brasileira de Normas Técnicas (ABNT). Substâncias Graxas - Determinação do Índice de Acidez; 1998.
American Oil Chemists’ Society (AOCS). In: Firestone D, editor. Official Methods and Recommended Practices of the American Oil Chemists’ Society: Fats, Oils and Lipid Related Analytical Methods. 6th ed. Champaign, IL: AOCS; 1993.
Jorge N, Soares BBP, Lunardi VM, Malacrida CR. Physico-chemical alterations of sunflower, corn and soybean oils in deep fat frying. Quim Nova. 2005;28:947–51.
Ribeiro APB, Grimaldi R, Gioielli LA, Gonçalves LAG. Zero trans fats from soybean oil and fully hydrogenated soybean oil: Physico-chemical properties and food applications. Food Res Int. 2009;42:401–10.
Candeia RA, Sinfrônio FSM, Bicudo TC, Queiroz N, Barros Filho AKD, Soledade LEB, Santos IMG, Souza AL, Souza AG. Influence of the storage on the thermo-oxidative stability of methyl and ethyl esters by PDSC. J Therm Anal Calorim. 2011;106:581–6.
Aued-Pimentel S, Kumagai EE, Kus MMM, Caruso MSF, Tavares M, Zenebon O. Trans fatty acids in refined polyunsaturated vegetable oils commercialized in the city of São Paulo, Brazil. Cienc Tecnol Aliment. 2009;29:646–51.
Neff WE, Selke E, Mounts TL, Rinsch W, Frankel EN, Zeitoun MAM. Effect of triacylglycerol composition and structures on oxidative stability of oils from selected soybean germplasm. J Am Oil Chem Soc. 1992;69:111–8.
BRASIL. Ministério da Saúde. Comissão Nacional de Normas e Padrões para Alimentos. Resolução no. 04/88. In: ASSOCIAÇÃO BRASILEIRA DAS INDÚSTRIAS DE ALIMENTAÇÃO, Compêndio da Legislação de Alimentos. São Paulo: ABIA, 2001. v. 1, p. 3.26.
Nogala-Kalucka M, Korczak J, Dratwia M, Lampart-Szczapa E, Siger A, Buchowski M. Changes in antioxidant activity and free radical scavenging potential of rosemary extract and tocopherols in isolated rapeseed oil triacylglycerols during accelerated tests. Food Chem. 2005;93:227–35.
Santos NA, Cordeiro AMTM, Damasceno SS, Aguiar RT, Rosenhaim R, Carvalho Filho JR, Santos IMG, Maia AS, Souza AG. Commercial antioxidants and thermal stability evaluations. Fuel. 2012;97:638–43.
Agência Nacional de Vigilância Sanitária (ANVISA). RDC no. 270, de 22 de setembro de 2005. Regulamento técnico para óleos vegetais, gorduras vegetais e creme vegetal. In: http://portal.anvisa.gov.br/wps/content/Anvisa+Portal/Anvisa/Inicio/Alimentos/Assuntos+de+Interesse/Legislacao. Accessed 14 Jan 2012.
Wang H, Liu F, Yang L, Zu Y, Wang H, Qu S, Zhang Y. Oxidative stability of fish oil supplemented with carnosic acid compared with synthetic antioxidants during long-term storage. Food Chem. 2011;128:93–9.
Mohdaly AAA, Sarhan MA, Mahmoudb A, Ramadan MF, Smetanska I. Antioxidant efficacy of potato peels and sugar beet pulp extracts in vegetable oils protection. Food Chem. 2010;123:1019–26.
Almeida-Doria RF, Regitano-D’arce MAB. Antioxidant activity of rosemary and oregano ethanol extracts in soybean oil under thermal oxidation. Cienc Tecnol Ali-ment. 2000;20:197–203.
Iqbal S, Bhanger MI. Stabilization of sunflower oil by garlic extract during accelerated storage. Food Chem. 2007;100:246–54.
Acknowledgements
The authors acknowledge the Brazilian agencies FAPEAM––Fundação de Amparo a Pesquisa do Estado do Amazonas, by the Ph.D. scholarship, and to MCT and FINEP.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cordeiro, A.M.T.M., Medeiros, M.L., Santos, N.A. et al. Rosemary (Rosmarinus officinalis L.) extract. J Therm Anal Calorim 113, 889–895 (2013). https://doi.org/10.1007/s10973-012-2778-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-012-2778-4