Abstract
This work shows the evaluation of three antioxidants (2,6-di-t-butyl-4-methylphenol (BHT)—synthetic antioxidant, hydrogenated cardanol (HC), and alkyl hydrogenated cardanol (AHC)—both derived from cashew nut shell liquid) on the thermo-oxidative stability of the soybean biodiesel. The antioxidants were added at concentrations of 200, 300, and 400 ppm, and the oxidative stability of the biofuel with and without antioxidants were investigated by thermogravimetric analysis (TG-DTG and IPDT) and Metrohm 743 Rancimat per the EN 14112 method. The results showed that all antioxidants contributed for the thermo-oxidative stability of the soybean biodiesel as follows: soybean biodiesel < soybean biodiesel + BHT < soybean biodiesel + HC < soybean biodiesel + AHC. In the Rancimat method, the results showed that the antioxidants influenced the biodiesel stability with an increase of at least 71 %.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biodiesel is an alternative fuel derived from vegetable oils, animal fats, or used frying oils [1–3]. This biofuel is made from transesterification of the lipid with a simple alcohol, such as methanol or ethanol, and can be used as a neat fuel, or it can be blended with petroleum or Fischer–tropsch diesel. Because many vegetable oils contain a significant amount of fatty acids with double bonds, oxidative stability is of concern, especially when storing biodiesel over an extended period of time [4, 5].
The oxidation stability of biodiesel is a function of the fatty acid composition, and decreases with higher contents of linoleic and linolenic acids [6–8]. This structural fact is a key to understanding both oxidative and thermal instability of these esters. In linoleic acids, polyunsaturated fatty acid chains contain two (C18:2) double bonds, while linolenic acids contain three (C18:3) double bonds. As a result, undesirable products, like gums, organic acids, and aldehydes, formed as a result of degradation, may cause injector and filter blockages resulting in engine problems [8, 9].
Despite its many advantages, the biodiesel has a poor thermo-oxidative stability. Its oxidation follows a free radical mechanism that starts with the abstraction of a hydrogen atom. The radical form (R·) rapidly reacts with oxygen to form a peroxy radical via a free radical chain reaction and the peroxy radical (ROO·) can gain a hydrogen atom to form a hydroperoxide (ROOH) [2, 10]. Once that, these oxidation reactions can cause a negative effect on the biodiesel performance, the biofuel industries have been using antioxidants to improve the quality of their esters. These additives, used in most formulations, control the oxygen-initiated degradation of the esters, protecting them from oxidation [11, 12].
Antioxidants are organic compounds that are added to oxidizable organic materials to retard oxidation and, in general, to prolong the useful life of the substrates [13]. These additives are classified as either radical trapping (chain breaking) or peroxide decomposing, terms that describe the mechanisms by which they function. The radical-trapping antioxidants, such as hindered phenols and secondary aromatic amines react with oxygen radicals (peroxy and alkoxy), while phosphites and phosphates function as peroxide decomposing by abstracting peroxidic oxygen from hydroperoxides and peroxides and reducing them [14, 15].
Among the radical-trapping antioxidants, the hindered phenol is one of the most important types of compound of this class. Thus, the present work evaluated three antioxidants of this class [2,6-di-t-butyl-4-methylphenol (BHT), hydrogenated cardanol (HC), and alkyl hydrogenated cardanol (AHC)] on the thermo-oxidative stability of soybean biodiesel [2]. Each antioxidant was added at concentrations of 200, 300, and 400 ppm. The oxidative stability of biodiesel with and without antioxidant was investigated by thermogravimetric analysis (TG-DTG) and Metrohm 743 Rancimat per the EN 14112 method. The integral procedure decomposition temperature (IPDT) was determined in this study too [16]. The antioxidants HC and AHC were derived from cashew nut shell liquid (CNSL), an important regional biomass.
Materials and methods
Materials
Hydrogenated cardanol (HC) and AHC were supplied by Laboratório de Inovações Tecnológicas e Especialidades Químicas–GRINTEQUI–Brazil. The HC and AHC (Fig. 1) were purified by column chromatography on silica gel using hexane as eluant [10]. Refined soybean oil was obtained in the local trade and the soybean biodiesel was synthesized by catalytic transesterification [2, 17]. The reagents and solvents were supplied by Aldrich (analytical grade).
Experimental
Synthesis of soybean biodiesel
Biodiesel from soybean oil was synthesized by the catalytic transesterification of soybean oil using methanol as aliphatic alcohol (molar ratio 1:8—oil:alcohol) and KOH as base [17]. The mixture was heated under reflux for 1 h and was monitored by thin-layer chromatography. After this time, the mixture was poured into a separating funnel and for difference of absolute density; the biodiesel was separated of the glycerin (major by-product). The light phase (rich in biodiesel) was separated, washed with hydrochloric acid solution (5 %) and water, dried with anhydrous sodium sulfate, and concentrated using rotary vacuum evaporator at 70 °C (±1 °C). The biodiesel was characterized by GC–MS, TG-DTG, and Rancimat per the EN 14112 method.
Formulation antioxidant/soybean biodiesel
The soybean biodiesel, obtained by the methyl route, was additivated with BHT, HC, and AHC antioxidants at concentrations of 200, 300, and 400 ppm by simple mixtures.
Physical measurements
The soybean biodiesel analysis was carried out using a GC–MS system. The configuration used here was the Shimadzu QP5050 equipped with a DB-1 column (30 m × 0.25 mm id × 0.25 μm film) used an oven temperature program that initiated data collection at a temperature of 100 °C and ramped at 10 °C min−1 to 300 °C, holding this temperature for the remaining duration of the data collection. Electron impact (EI, 70 eV) mode was used and sample of 1 μL was injected into the column. Table 1 provides an overview of all the instrument parameters.
Thermoanalytical measurements were obtained in a Mettler Toledo TGA/SDTA85 using alumina pans and a heating rate of 10 °C min−1 in the temperature range 25–800 °C, and mass of approximately 10 mg. The samples were carried out in synthetic air atmosphere (50 mL min−1).
The oxidative stabilities of the samples were determined by a Metrohm 743 Rancimat per the EN14112 method. In this test, a 10 L h−1 stream of dry air is bubbled into 3 g samples maintained at 110 °C, volatile oxidation products are carried through the detector chamber containing deionized water. The change in conductivity is measured and recorded every 36 s. The increase in conductivity is measured as a function of time until maximal change which reflects the oxidative stability.
Results and discussion
GC–MS, TG-DTG, IPDT, and Rancimat—soybean biodiesel
Figure 2 presents an identified chromatogram with fatty acid methyl esters present in the soybean biodiesel. According to the results, the chromatogram denotes the preponderance of methyl palmitate, methyl linoleate, methyl cis-9-octadecanoate, and methyl octadecanoate in the mixture of methyl esters (Table 2). The GC–MS peak report showed that the overall amount of methyl esters was 100 %, what confirms the efficiency of the purification process carried out after the biodiesel synthesis, once that, according to the European Standard EN 14103, the overall ester content must be higher than 96.5 % [1, 9].
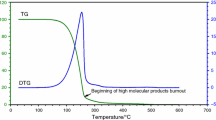
The TG-DTG curves of soybean biodiesel show that the sample is thermally stable up to 175 °C (Fig. 3), between 175 and 350 °C, the DTG curve shows mass loss occurring in one degradation event. The Figs. 4, 5, and 6 show the DTG curves of the formulations of antioxidant/soybean biodiesel. According to the results, it is noteworthy that soybean biodiesel exhibits oxidative stability smaller than the formulations (T max). Table 3 summarizes the thermal parameters of soybean biodiesel with and without antioxidants. This thermo-oxidative study reported the relative stabilities of the samples, which are as follows: soybean biodiesel < soybean biodiesel + BHT 200 ppm < soybean biodiesel + HC 200 ppm < soybean biodiesel + AHC 200 ppm; for the other proportion of 400 ppm, the samples proceeded with the same behavior, when evaluated the second event. This result is compatible with the literature of the hindered phenolic antioxidants [18–20], which says that their bulky substituents influence the specificity of the phenols by blocking phenoxyl radicals from abstracting hydrogen atoms from organic substrates [21], and AHC possesses two substituents in its ring, in the ortho and meta positions. The first and second events are due to volatilization of methyl esters.
The values of integral procedural decomposition temperature (IPDT) calculated by Doyle’s [16] method are in the range of 226–260 °C. These values represent an overall thermal stability of the materials. The IPDT is calculated as follows:
where A* is the area ratio of total experimental curve divided by total TG thermogram [(D1 + D2)/(D1 + D2 + D3)], K* is the coefficient [(D1 + D2)/(D1)], T i the initial experimental temperature and T f the final experimental temperature. Figure 7 shows a schematic representation of D1, D2, and D3 for calculation of the parameters A* and K*. According to the results (Table 3), the formulations that present the better stabilities were: soybean biodiesel + AHC 200 ppm and soybean biodiesel + AHC 400 ppm. This result confirms once again the potentiality of the AHC. On the other hand, the BHT presented low stability potential, probably due to its complete vaporization [5, 22].
For the induction period (IP) determination, the samples were analyzed by Rancimat (EN14112) method. This accelerated oxidation test is used frequently for predicting the oxidative stability (shelf life) of fats, oils, and biofuels, and the efficacy of antioxidants for increasing their stabilities [2, 13] (Fig. 8). Table 4 summarizes the parameters of oxidative stability of the samples. In this procedure, the soybean biodiesel was additivated with BHT, HC, and AHC antioxidants at concentration of 300 ppm, an average of the extreme values of 200 and 400 ppm.
The addition of the antioxidants, HC, AHC, and BHC in the soybean biodiesel at concentration of 300 ppm, influenced the biodiesel stability with an increase of at least 71 % (HC antioxidant), as determined by the Rancimat method (Table 4). The IP of the soybean biodiesel increased 101.7 % when 300 ppm of BHT was added, and the offered protection by AHC when 300 ppm was added was of 111.9 %. According to the results, although the induction periods of the samples have presented values below 6 h, the addition of the antioxidants in low concentrations in the biodiesel was very important for its oxidative stability. Once that this work presented promising results, the authors are working in formulations with the use of larger concentrations of the antioxidants HC, AHC, and BHT. The results will be presented in another paper.
Conclusions
The presence of methyl esters from the unsaturated fatty acids in the soybean biodiesel denotes its low induction period. In spite of this result, the addition of the antioxidants HC, AHC, and BHC in the soybean biodiesel at concentrations of 200, 300, and 400 ppm, influenced the biodiesel stability. The TG-DTG curves of soybean biodiesel shows that the sample is thermally stable up to 175 °C and the thermo-oxidative study reported that the relative stability of the samples are as follows: soybean biodiesel < soybean biodiesel + BHT < soybean biodiesel + HC < soybean biodiesel + AHC. The results obtained by TG-DTG, IPDT, and Rancimat method were consistent in terms of the potentiality of AHC. The TG-DTG and IPDT were used as alternative techniques to determination of the oxidative stability of the soybean biodiesel.
References
Tavares MLA, Queiroz N, Santos IMG, Souza AL, Cavalcanti EHS, Barros AKD, Rosenhaim R, Soledade LEB, Souza AG. Sunflower biodiesel. Use of P-DSC in the evaluation of antioxidant efficiency. J Therm Anal Calorim. 2011;106:575–9.
Souza FHN, Almeida LR, Batista FSCL, Rios MAS. UV-visible spectroscopy study of oxidative degradation of sunflower biodiesel. Energ Sci Technol. 2011;2:56–61.
Ferrari RA, Souza WL. Avaliação da estabilidade oxidativa de biodiesel de óleo de girassol com antioxidantes. Quim Nova. 2009;32:106–11.
Jain S, Sharma MP. Stability of biodiesel and its blends: a review. Renew Sust Energ Rev. 2010;2:667–8.
Maia ECR, Borsato D, Moreira I, Spacino KR, Rodrigues PRP, Gallina AL. Study of the biodiesel B100 oxidative stability in mixture with antioxidants. Fuel Process Technol. 2011;92:1750–5.
Dantas MB, Conceição MM, Fernandes VJ Jr, Santos NA, Rosenhaim R, Marques ALB, Santos IMG, Souza AG. Thermal and kinetic study of corn biodiesel obtained by the methanol and ethanol routes. J Therm Anal Calorim. 2007;87:835–9.
Rodrigues FMG, Souza AG, Santos IMG, Bicudo TC, Silva MCD, Sinfrônio FSM, Vasconcelos AFF. Antioxidative properties of hydrogenated cardanol for cotton biodiesel by P-DSC and UV/VIS. J Therm Anal Calorim. 2009;97:605–9.
Oetterer M, D’Arce MABR, Spoto M. Fundamentos de ciência e tecnologia de alimentos. São Paulo: Editora Manole; 2006.
Candeia RA. Biodiesel de Soja: Síntese, Degradação e Misturas Binárias. Doctoral Thesis. Organic Chemistry Department, Federal University of Paraíba, Brazil; 2008.
Maria ARF, Síntese e Aplicabilidade de Antioxidantes derivados do Cardanol hidrogenado. Doctoral Thesis. Organic and Inorganic Chemical Department, Federal University of Ceará, Brazil; 2008.
Rios MAS, Mazzetto SE. Effect of organophosphate antioxidant on the thermo-oxidative degradation of a mineral oil. J. Therm. Anal. Calorim. 2011. doi:10.1007/s10973-011-2160-y.
Maria ASR, Francisco AMS, Selma EM. Study of antioxidant properties of 5-n-pentadecyl-2-tert-amylphenol. Energ Fuel. 2009;23:2517–22.
Chaithongdee D, Chutmanop J, Srinophakun P. Effect of antioxidants and additives on the oxidation stability of jatropha biodiesel. Kasetsart J (Nat Sci). 2010;44:243–50.
Polavka J, Paligová J, Cvengros J, Simon J. Oxidation stability of methyl esters studied by differential thermal analysis and Rancimat. J Am Oil Chem Soc. 2005;82:519–24.
Rios MAS, Santiago SN, Lopes AAS, Mazzetto SE. Antioxidative Activity of 5-n-pentadecyl-2-tert-butylphenol stabilizers in mineral lubricant oil. Energ Fuel. 2010;24:3285–91.
Doyle CD. Estimating thermal stability of experimental polymers by empirical thermogravimetric analysis. Anal Chem. 1961;33:77–9.
Almeida LR, Silva AL, Souza FHN, Hidalgo AA, Vega ML, Cunha HN, Rios MAS. Evaluation of antioxidant potentiality of compounds derived from biomass for application in biodiesel from soybean oil. Quim Brazil 2011; (In press).
Ohkatsu Y, Nishiyama T. Phenolic antioxidants-effect of ortho-substituents. Polym Degrad Stabil. 2000;67:313–8.
Wright JS, Johnson ER, DiLabio GA. Predicting the activity of phenolic antioxidants: theoretical method, analysis of substituent effects, and application to major families of antioxidants. J Am Chem Soc. 2001;123:1173–83.
Ohkatsu Y, Ishikawa S, Tobita E. Consideration on the effect of ortho-substituents of phenols by semiempirical molecular orbital method MOPAC. Polym Degrad Stabil. 2000;67:541–5.
Lai JT. Totally hindered phenols. 2,6-di-t-butyl-4-(1,1-dialkyl-1-acetamide)-phenols and their persistent phenoxy radicals. Tetrahedron Lett. 2001;42:557–60.
Santos NA, Cordeiro AMTM, Damasceno SS, Aguiar RT, Rosenhaim R, Filho JRC, Santos IMG, Maia AS, Souza AG. Commercial antioxidants and thermal stability evaluations. Fuel. 2012;97:638–43.
Acknowledgements
The authors acknowledge CNPq (Process No. 476568/2010-2) and FAPEPI for the financial support, and the Organic and Inorganic Chemistry Department of Federal University of Ceará by chemical and thermal analyses.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rios, M.A.S., Santos, F.F.P., Maia, F.J.N. et al. Evaluation of antioxidants on the thermo-oxidative stability of soybean biodiesel. J Therm Anal Calorim 112, 921–927 (2013). https://doi.org/10.1007/s10973-012-2650-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-012-2650-6