Abstract
Stoichiometric mixture of CuO and α-Fe2O3 milled in air up to 30 h was subjected to different heat treatments. The evolution of the heat treated milled powders was investigated by X-ray diffraction (XRD). The CuFe2O4 was partially obtained by milling, the material consisting in a mixture of phases. By applying different heat treatments in air and in vacuum, for 2–6 h, in 500–800 °C temperature range the phases composition of the milled samples is changed. A heat treatment at 500 °C in vacuum favours the formation of delafossite (CuFeO2) and tenorite (CuO) phases. If the same heat treatment is made in air, the CuFe2O4 phase formation with a cubic structure is favoured. Differential scanning calorimetry (DSC) investigation realised in Ar atmosphere revealed two large exothermic peaks. The first one is associated with the formation of the delafossite and tenorite phases and the second one with the formation of CuFe2O4. The XRD patterns of the samples subjected to the DSC measurements present maxima corresponding to the delafossite and cuprospinel (CuFe2O4) phases. For the heat treatment at 600 °C in air the phases present in the sample are the same as for the annealing performed at 500 °C: CuFe2O4, α-Fe2O3 and CuO. The heat treatment in air at 800 °C leads to the complete reaction between the different phases and the formation of CuFe2O4 phase in whole the sample volume. The CuFe2O4 ferrite crystallises after this heat treatment in two crystal systems: cubic and tetragonal.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The spinel ferrites, MeFe2O4 (where Me is a metallic element or a group of metallic elements), are from decades in the centre of many researches all over the world due to the different and interesting properties which they own if they are synthesized in vary form and size such as: powder and pellet or nanocrystalline and nanosized state [1–7]. The ferrites are synthesized classically by ceramic method which involves the reaction of the oxides (MeO and Fe2O3) precursors in solid state at relatively high temperature [1]. Beside the classical ceramic route many other routes are used such as: sol–gel [8, 9], co-precipitation [10, 11] or mechanosynthesis (mechanochemical route) [12–14]. In order to synthesize ferrites so called combined routes have also been reported amongst these methods one can mention co-precipitation/mechanical milling [15]. The synthesis of ferrites using these routes is achieved starting from various precursors. Using those methods the state of material is changed, the material is in nanocrystalline or nanostructured state and the properties of the material are changed. The spinel ferrites crystallize in cubic structure, space group—Fd3m. They are ionic compounds in which the Me2+ cations and Fe3+ cations are surrounded by O2− anions in two types of crystallographic positions corresponding to tetrahedral and octahedral sites [1]. One of the most interesting ferrites is copper ferrite, CuFe2O4. The copper–iron oxide (CuFe2O4), as all the spinel ferrites, crystallizes in the cubic spinel structure in some condition and is called cuprospinel. Beside this system, there is another possibility for crystallisation of this oxide, the tetragonal structure, spatial group—I41amd. The crystallisation of the CuFe2O4 in a structure or another depends upon the synthesis condition [1, 16]. Typically, for the copper–iron oxide, a synthesis by ceramic route followed by a quench from above 760 °C, leads to a cubic spinel structure with the Cu2+ cations in octahedral sites and Fe3+ cations distributed between the tetrahedral and octahedral sites [1]. If the samples are slowly cooled it becomes tetragonal and the copper and iron cations occupy similar crystallographic sites as for the cubic spinel. In both cases the structure of CuFe2O4 is an inverse spinel [16]. The synthesis of the CuFe2O4 using mechanochemical synthesis route starting from a stoichiometric mixture of oxides, CuO and α-Fe2O3, has some drawbacks since the Cu–O–Fe system has a large reversibility [17]. The formation of a CuO–Fe2O3 solid solution between the starting oxides mixture has been reported to occur during milling, the solid solution has corundum structure [18, 19]. The synthesis of the copper ferrite was achieved by mechanical milling of the precursors oxides followed by annealing treatment [20].
This article presents results of the investigation of the influence of the heat treatments condition on the formation of CuFe2O4 from mechanical milled precursor oxides, CuO and α-Fe2O3.
Experimental
A stoichiometric mixture of high purity commercial oxides powders (Alpha Aesar), copper oxide (CuO—tenorite) and iron oxide (α-Fe2O3—hematite) was used as starting sample (ss) for the reactive milling (RM). The stainless steel vials and balls were used. The milling conditions are similar with the ones used previously and described elsewhere [21]. After this milling, in order to obtain CuFe2O4 several heat treatments have been applied. The heat treatments were realized in air and in vacuum at 350–800 °C temperature range and for times between 2 to 6 h.
The formation of copper ferrite by milling and heat treatment and the evolution of the other phases during milling have been investigated by X-ray diffraction (XRD). A Siemens D5000 diffractometer which operates in reflection with Co Kα radiation (λ = 1.7903 Å) was used.
The differential scanning calorimetry (DSC) measurements were realized using NETZSCH–DSC 404S apparatus and as reference an alumina sample. We used a heating/cooling speed of 10 °C/min and the temperature range was between 25 and 1,000 °C. The measurements were performed in Ar atmosphere.
Results and discussion
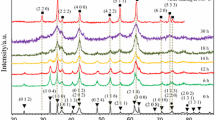
The XRD patterns of the ss (stoichiometric mixture of CuO and α-Fe2O3), as-milled samples (4, 8, 12, 16, 20, 24 and 30 h of milling) and the milled and subsequently annealed (500 °C/4 h in vacuum) samples are shown in the Fig. 1. For the as-milled samples, the phases observed in the material are very different depending upon the milling time, as reported in our previous study [19]. After 4 h of milling a mixture of CuO–α-Fe2O3 solid solution and cubic CuFe2O4 phase is observed. These results confirm the formation of a solid solution between precursor oxides as was reported also in [18, 19]. For the 8, 12 and 16 h milled samples, another phase is formed beside the solid solution and copper ferrite, cuprite—Cu2O. The XRD patterns of the samples milled for 20, 24 and 30 h contain the (110) Bragg reflection of elemental iron. This is due to the powder contamination by the vials and balls during milling [21, 22]. One cannot exclude that the powder contamination occurs even earlier, but in such a case the amount of elemental iron is too small to be detected by XRD (that is to say below a few percents). Further milling, for 24 and 30 h, leads to the dissociation of Cu from the oxides as can be seen from the appearance of Bragg peaks attributed to elemental copper in the diffraction patterns. After annealing at 500 °C for 4 h in vacuum new phases are formed in the material. For the 4 h milled and subsequently annealed sample the diffraction pattern exhibit new maxima assigned to the delafossite CuFeO2 and tenorite CuO phases. For CuFeO2 the following reflection are noticed in the diffraction pattern: (222), (100), (110), (211), (332), (110), (433) and (443). The most intense Bragg peak of this oxide, attributed to the (110) reflection, is overlapping with the (311) one of CuFe2O4 (110) of α-Fe2O3 and (110) and (002) of CuO. The (111) and (220) Bragg peaks of CuO are very close and cannot be distinguished from one another. The delafossite phase is forming from the transformation of a part of solid solution CuO–Fe2O3 in CuFeO2. The transformation of CuFe2O4 into CuFeO2 can be excluded due to the fact that the (220) reflection of the copper ferrite (which is not the most intense) is present in the diffraction patterns and is more intense compared with the as-milled sample. The transformation of the solid solution of CuO–Fe2O3 into CuFeO2 does not respect the stoichiometry. In CuO–Fe2O3 solid solution the expected ratio between the metallic elements and oxygen (assuming that there was no release of oxygen during milling) is 3:4, whereas in the delafossite phase this ratio is 1:1. This suggests also a release of oxygen during the milling. Another explanation is that the delafossite phase which is formed has metallic vacancies, \( {\text{Cu}}_{ 1- x} {\text{Fe}}_{ 1- y} {\text{O}}_{ 2} \) (where x represent the Cu vacancies and y the Fe vacancies). One can remark that the formed oxide probably does not respect the Cu:Fe ratio of 1:1 expected for CuFeO2 delafossite due to the excess iron cations which are provided by the iron richer solid solution CuO–Fe2O3. Practically the chemical formula of the delafossite formed here can be written as: \( {\text{Cu}}_{ 1- x - s} {\text{Fe}}_{ 1- y + s} {\text{O}}_{ 2} \), where s represent the Cu cations replaced by Fe cations. The formation of CuO is occurs most probably from the decomposition of the CuO–Fe2O3 solid solution.
It is worth to mention that the CuO Bragg peak can be better seen by refining the structure (removing the stresses and/or defects and increasing the crystallite size) of the non-reacted CuO.
For the 8, 12, 16, 20 and 24 h milled and annealed samples the same phases are observed in the obtained material as for the 4 h milled and annealed sample. For these milling times Cu2O was present in the as-milled sample, whereas this phase disappears upon annealing, this indicates that this oxide reacted during the heat treatment. Similarly, the elemental Fe and Cu observed for the long milling times (20, 24 and 30 h) have also reacted during the annealing treatment. For the 30 h milled sample the (111) and (220) Bragg peaks of CuO are much less intense in comparison with the other samples and only a shoulder of the (111) diffraction line of Cu2O is noticed in Fig. 1. We mentioned above as suggested in our previous work [19] that a release of some oxygen during milling from the processed powder is also possible. The presence of the Cu2O in the 30 h milled and subsequent annealed sample confirms somehow this assumption. Due to the lack of oxygen for the 30 h milled and subsequent annealed sample the amount of CuO is less and a small amount of Cu2O remains in the sample. We interpret this as resulting of a lack of oxygen to oxidize the Cu2O.
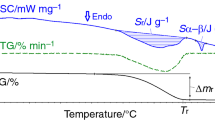
The DSC curves recorded during heating up to 1,000 °C in Ar atmosphere for the CuO + α-Fe2O3 samples milled for 4, 8, 12, 20 and 24 h are presented in the Fig. 2. For all the samples the heat released up to 350 °C is associated with the release of the internal stresses induced in the samples by the milling process. For the 4 and 8 h milled samples, two large exothermic peaks are remarked. The first exothermic peak is associated with the formation of the CuFeO2 and CuO and the second peak with the formation of the CuFe2O4. The XRD pattern recorded for the milled and annealed at 500 °C/4 h samples indicate the formation of delafossite at this temperature. The first exothermic peak started after 350 °C and is finished at about 707 °C for the 4 h milled sample and at 711 °C for the 8 h milled samples. The formation of the CuFe2O4 phase begins immediately after the end of the delafossite formation and it is finished at about 870 °C. The peak associated with the formation of CuFeO2 ended at higher temperature (712, 715 and 720 °C for sample milled for 12, 20 and 24 h respectively) for these samples compared with the samples milled for 4 or 8 h. This can be due to the existence of several phases in the milled material. Furthermore, the formation of the CuFe2O4 seems to begin and end at higher temperatures; this could be also the results of the lack of oxygen. During the heating the CuFeO2 it is transformed in copper ferrite with oxygen vacancies—CuFe2O4−γ (where γ represents the oxygen vacancies). The formation of the copper spinel with oxygen vacancies was previously reported by Goya and Rechenberg [17]. The other exothermic peak present in the sample milled for 12, 20 and 24 h is associated with the recrystallisation of the Fe2O3 and is observed at about 593 °C for the 12 and 20 h milled samples recrystallisation and at 596 °C for the 24 h milled sample [23]. The recrystallisation process of the hematite is probably also present in the 4 and 8 h milled samples but is masked due to the presence of the other processes. The recrystallisation process is more obvious for these samples due to the partial decomposition of the solid solution during milling, and consequently the recrystallisation is more easy. The association of the heat released up to 350 °C with the stress release is confirmed by the XRD patterns recorded for the samples milled and subsequently annealed at 350 °C/4 h in vacuum. In these milled and annealed samples (350 °C/4 h) there is no evidence of formation of CuFeO2 and the diffraction peaks are better defined and less broadened.
The assignment of the second exothermic peak to the formation of CuFe2O4 is sustained also by XRD investigation made after the DSC measurements. In the Fig. 3 are presented the XRD patterns recorded for the ss, as well as for the as-milled for 4, 16 and 30 h subjected to DSC measurements up to 1,000 °C in Ar samples. During the DSC measurement in the ss and in the 16 and 30 h milled samples are formed three phases: cubic CuFe2O4, CuFeO2 and α-Fe2O3. The 4 h milled and subjected to DSC sample present two phases: cubic CuFe2O4 and CuFeO2. The existence of the Fe2O3–CuO solid solution in this sample is favourable to the formation of the cuprospinel—CuFe2O4. The presence of the delafossite after the DSC treatment in these samples is due to the lack of oxygen which favours the formation of this phase instead of cuprospinel. The amount of CuFe2O4 seems to be higher in the 4 h milled sample subjected to DSC compared with the amount of CuFeO2 whilst the ratio between the intensities of the most intense Bragg peak of the cubic spinel copper ferrite to that of the most intense maxima of the delafossite is 1.47. For the ss subjected to DSC this ratio is 0.96, the amount of each phase seems to be the same (see the inset of the Fig. 3). For the 4 h milled sample the major part of the Fe2O3–CuO solid solution is transformed into CuFe2O4 during DSC treatment and for the 16 and 30 milled sample, after DSC the amount of the hematite is higher, the formation of Cu2O and the presence of elemental Cu and Fe leads to a high amount of Fe2O3 after DSC. The copper ferrite formed after the DSC treatment exhibits cubic symmetry for all the studied samples.
Changing the annealing atmosphere from vacuum to air and maintaining the temperature of the treatment at 500 °C and the annealing time at the same 4 h, leads to different phases present in the material. In the Fig. 4 are shown the XRD patterns of the ss, of the as-milled samples (4, 8, 12, 16, 20, 24 and 30 h of milling) and of the samples milled and annealed at 500 °C/4 h in air. During this heat treatment in air there are no evidences of the formation of the delafossite phase. For all the samples milled and annealed at 500 °C/4 h in air, the samples are containing the following phases: CuFe2O4, Fe2O3 and CuO. The CuO phase is formed from the dissolution of the Fe2O3–CuO and from the crystallisation of the amorphous CuO for the sample milled 4 and 8 h. For the rest of the sample the formation of the CuO can be attributed also to the oxidation of the Cu2O:
For the samples milled 24 and 30 h another possibility of the formation of the tenorite is the copper provided by the reduction of the Cu2O [19]:
In addition, one must keep in mind that during this heat treatment the excess of Fe, coming from contamination during milling process, will react to form oxide. The Bragg peaks of CuFe2O4 were attributed to the cubic spinel structure.
Increasing the temperature of the heat treatment at 600 °C and reducing the treatment time at 2 h we obtained an increase of the amount of the CuFe2O4. In Fig. 5a are presented XRD patterns recorded for the ss, the 16 and 30 h milled samples and the samples milled for 16 and 30 h followed by heat treated at 600 °C/2 h in air samples. In the same figure are presented the XRD patterns recorded for the 16 h milled and heat treated in air samples at 600 and 800 °C/6 h, respectively. For both heat treated samples at 600 °C for 2 h, the phases present in the material are the same as for the treatment performed at 500 °C for 4 h in air: CuFe2O4, Fe2O3 and CuO. This heat treatment is favourable to the formation of the cubic CuFe2O4; the Bragg peaks corresponding to this phase are better defined due to the higher annealing temperature.
a XRD patterns of the ss, 16 and 30 h milled samples, 16 and 30 h milled and heat treated at 600 °C/2 h in air samples, 16 h milled and heat treated at 600 and 800 °C/6 h samples and b fit and deconvolution of the experimental XRD pattern of the tetragonal (103) and (211) and cubic (311) reflections of CuFe2O4 for the 16 h milled and heat treated at 800 °C for 6 h. 1 Experimental points, 2 fitted curve of experimental points, 3 (103) peak of tetragonal CuFe2O4, 4 (211) peak of tetragonal CuFe2O4, 5 (311) Bragg peak of cubic CuFe2O4, 6 positions of the Bragg peaks, 7 difference between the experimental points and the corresponding fit
For example, the (220) diffraction line of the copper spinel was difficult to identify in the diffraction pattern of the sample milled 30 h and heat treated at 500 °C/4 h in air. After this treatment at 600 °C, the (220) maximum of the copper spinel is of similar intensity as the most intense maxima of the hematite. The CuO maxima are reduced in intensity as a result of the reaction of this phase by reacting with Fe2O3. The higher milling time is also more favourable to the formation of this phase, the maxima of the copper spinel are much more intense in comparison with the other phases in the diffraction pattern of the 30 h milled sample compared with the 16 h milled sample.
In order to increase the amount of CuFe2O4, the temperature and the duration of the heat treatment were increased. By increasing the heat treatment time from 2 to 6 h for the 16 h milled sample the amount of the CuFe2O4 increases, the cubic spinel XRD lines are more intense comparison with the other oxides lines. A new Bragg peak is observed in the diffraction pattern after this heat treatment. This corresponds to the (103) diffraction line of CuFe2O4 with tetragonal structure. During heat treatment a part of CuFe2O4 with cubic structure is transformed into CuFe2O4 with tetragonal structure. Another possibility of the tetragonal CuFe2O4 formation is the reaction of a part of the Fe2O3 and CuO amount during annealing in the range of 2–6 h (after 2 h of heat treatment the tetragonal structure was not evidenced). By increasing the temperature of the heat treatment at 800 °C and maintaining the treatment duration at 6 h for the 16 h milled sample only CuFe2O4 was obtained. During this heat treatment the solid solution Fe2O3–CuO and the Cu2O fully reacts in the presence of the oxygen forming the CuFe2O4. After this heat treatment the obtained CuFe2O4 powder is also crystallised in two crystal symmetries: cubic and tetragonal. The maxima of both CuFe2O4—tetragonal and CuFe2O4—cubic are present in the diffraction pattern, but the maxima of the tetragonal phase are more enhanced compared with the maxima of the cubic phase and the amount of the material crystallised in the tetragonal system is larger. The fit and deconvolution of the tetragonal CuFe2O4 (103) and (211) lines and cubic CuFe2O4 (311) reflection for the 16 h milled and subsequently annealed at 800 °C/6 h is presented in Fig. 5b. The ratio between the most intense peak of the tetragonal structure (211) and the most intense maxima of cubic structure (311) is 1.6, which means an amount of 62% tetragonal CuFe2O4 and respectively 38% cubic CuFe2O4. The copper ferrite structure seems to be sensitive to this heat treatment temperature and mainly changes its crystal structure type from cubic to tetragonal. For the milled samples, the preferred crystallographic structure of the copper ferrite seems to be cubic symmetry [24]. Similar behaviour has been reported by Berbenni et al. [20], which obtained the tetragonal structure of CuFe2O4 after the heat treatment at 750–800 °C of the mechanically milled precursors. The change of crystallographic system has been also observed by Stewart et al. [25] for a copper ferrite synthesized by co-precipitation. Indeed they observed a heat treatment induced change from cubic to tetragonal.
The lattice parameter calculated for both tetragonal and cubic symmetries are in good correlation with the previously reported results [1, 26, 27]. We obtained a value for the lattice parameter of 8.369 Å for the cubic structure. This value of the lattice parameter is very close to the one from the JCPDS file 77-0010, 8.37 Å. For the tetragonal structure the value for the lattice parameters, where c = 8.575 Å and a = 5.850 Å and these values are in good agreement with the JCPDS file 34-0425, c = 8.630 Å and a = 5.844 Å. The difference between the calculated values of the lattice parameters and the ones from the reference file could be related to different cation distribution in the spinel structure. Indeed, if the obtained copper ferrite has a cations distribution which do not respect the arrangement of an inverse spinel (Cu2+ cations in octahedral sites) it is possible to have a mixed spinel structure (part of Cu2+ cations in tetragonal sites). In this case the formula of the copper spinel taking into account the oxygen release and mixed spinel structure becomes: \( ( {\text{Cu}}_{{ 1- {{\updelta}}}} {\text{Fe}}_{{{\updelta}}} )^{\text{tetra}} ( {\text{Fe}}_{{ 2- {{\updelta}}}} {\text{Cu}}_{{{\updelta}}} )^{\text{octa}} {\text{O}}_{{ 4- {{\upgamma}}}} \), where γ represent the oxygen vacancies.
Conclusions
The influence of the heat treatment, in vacuum and in air, over the formation of the CuFe2O4 from the mechanically milled precursor oxides has been evidenced. The RM of the stoichiometric mixture of precursor oxides (CuO and α-Fe2O3) leads to the formation of various phases in the as-milled samples, depending upon milling time. The heat treatment in vacuum at 500 °C is favourable to the formation of the delafossite phase, also during this heat treatment is forming CuO. The DSC analyses in Ar revealed two exothermic peaks, one associated with the formation of CuFeO2 and CuO, and the second at temperature higher than 700 °C associated with the formation of CuFe2O4. The heat treatments in air are more favourable to the formation of the CuFe2O4 due to the oxygen contribution. After the heat treatment at 500 and 600 °C the sample α-Fe2O3 and CuO phases beside the CuFe2O4 phase. For the samples milled for longer milling time the amount of the CuFe2O4 which is forming is bigger. For heat treatment temperature of 800 °C a complete reaction between the precursors was obtained and in the material the CuFe2O4 phase is the only one observed. The CuFe2O4 formed after this heat treatment has two types of symmetries: tetragonal (in majority) and cubic. The lattice parameter obtained for the tetragonal CuFe2O4 indicates that a is lower and c is larger comparison with the reference and this was attributed to the cation distribution.
References
Cullity BD, Graham CD. Introduction to magnetic materials. 2nd ed. Hoboken: IEEE Press; 2009.
Goldman A. Modern ferrite technology. 2nd ed. Pittsburgh: Springer; 2006.
Tkáčová K, Šepelák V, Šetevulová N, Boldyrev VV. Structure–reactivity study of mechanically activated zinc ferrite. J Solid State Chem. 1996;123:100–8.
Chinnasamy CN, Narayanasamy A, Ponpandian N, Joseyphus RJ, Chattopadhyay K, Shinoda K, Jeyadevan B, Tohji K, Nakatsuka K, Guérault H, Greneche JM. Structure and magnetic properties of nanocrystalline ferrimagnetic CdFe2O4 spinel. Scr Mater. 2001;44:1411–5.
Kodama RH, Berkowitz AE, McNiff EJ Jr, Foner S. Surface spin disorder in NiFe2O4 nanoparticles. Phys Rev Lett. 1996;77(2):394–7.
Goya GF. Handling the particle size and distribution of Fe3O4 nanoparticles through ball milling. Solid State Commun. 2004;130:783–7.
Thapa D, Kulkarni N, Mishra SN, Paulose PL, Ayyub P. Enhanced magnetization in cubic ferrimagnetic CuFe2O4 nanoparticles synthesized from a citrate precursor: the role of Fe2+. J Phys D Appl Phys. 2010;43:195004 (1–5).
Oliver SA, Hamdeh HH, Ho JC. Localized spin canting in partially inverted ZnFe2O4 fine powders. Phys Rev B. 1999;60(5):3400–5.
Srivastava M, Chaubey S, Ojha AK. Investigation on size dependent structural and magnetic behavior of nickel ferrite nanoparticles prepared by sol–gel and hydrothermal methods. Mater Chem Phys. 2009;118:174–80.
Gomes JA, Sousa MH, Tourinho FA. Rietveld structure refinement of the cation distribution in ferrite fine particles studied by X-ray powder diffraction. J Magn Magn Mater. 2005;289:184–7.
Hankare PP, Kadam MR, Patil RP, Garadkar KM, Sasikala R, Tripathi AK. Effect of zinc substitution on structural and magnetic properties of copper ferrite. J Alloys Compd. 2010;501:37–41.
Ding J, McCormick PG, Street R. Formation of spinel Mn-ferrite during mechanical alloying. J Magn Magn Mater. 1997;171:309–14.
Bid S, Pradhan SK. Characterization of crystalline structure of ball-milled nano-Ni–Zn-ferrite by Rietveld method. Mater Chem Phys. 2004;84:291–301.
Verdier T, Nivoix V, Jean M, Hannoyer B. Characterization of nanocrystalline Mn–Zn ferrites obtained by mechanosynthesis. J Mater Sci. 2004;39:5151–4.
Manova E, Tsoncheva T, Paneva D, Popova M, Velinov N, Kunev B, Tenchev K, Mitov I. Nanosized copper ferrite materials: mechanochemical synthesis and characterization. J Solid State Chem. 2011;184:1153–8.
Stewart SJ, Tueros MJ, Cernicchiaro G, Scorzelli RB. Magnetic size growth in nanocrystalline copper ferrite. Solid State Commun. 2004;129:347–51.
Goya GF, Rechenberg HR. Reversibility of the synthesis–decomposition reaction in the ball-milled Cu–Fe–O system. J Phys Condens Matter. 1998;10:11829–40.
Jiang JS, Yang XL, Gao L, Guo JK. Nanostructured CuO–α-Fe2O3 solid solution obtained by high-energy ball milling. Mater Sci Eng A. 2005;392:179–83.
Marinca TF, Chicinaş I, Isnard O. Synthesis, structural and magnetic characterization of nanocrystalline CuFe2O4 as obtained by a combined method reactive milling, heat treatment and ball milling. Ceram Int. 2011. doi:10.1016/j.ceramint.2011.10.026.
Berbenni V, Marini A, Milanese C, Bruni G. Solid state synthesis of CuFe2O4 from Cu(OH)2·CuCO3–4FeC2O4·2H2O mixtures: mechanism of reaction and thermal characterization of CuFe2O4. J Therm Anal Calorim. 2010;99:437–42.
Marinca TF, Chicinaş I, Isnard O, Pop V, Popa F. Synthesis, structural and magnetic characterization of nanocrystalline nickel ferrite-NiFe2O4 obtained by reactive milling. J Alloys Compd. 2011;509:7931–6.
Marinca TF, Chicinaş I, Isnard O, Pop V. Structural and magnetic properties of nanocrystalline ZnFe2O4 powder synthesized by reactive ball milling. Optoelectron Adv Mater Rapid Commun. 2011;5(1–2):39–43.
Hofmann M, Campbell SJ, Kaczmarek WA. Mechanochemical treatment of α-Fe2O3: a neutron diffraction study. Appl Phys A. 2002;74:S1233–5.
Jiang JZ, Goya GF, Rechenberg HR. Magnetic properties of nanostructured CuFe2O4. J Phys Condens Matter. 1999;11:4063–78.
Stewart SJ, Mercader RC, Vandenberghe RE, Cernicchiaro G, Scorzelli RB. Magnetic anomalies and canting effects in nanocrystalline spinel copper ferrites CuxFe3−xO4. J Appl Phys. 2005;97:054304 (1–6).
Deraz NM. Size and crystallinity-dependent magnetic properties of copper ferrite nano-particles. J Alloys Compd. 2010;501:317–25.
Wu X, Zhou K, Wu W, Cui X, Li Y. Magnetic properties of nanocrystalline CuFe2O4 and kinetics of thermal decomposition of precursor. J Therm Anal Calorim. 2011. doi:10.1007/s10973-011-2104-6.
Acknowledegements
This study was supported by CNCSIS—UEFISCSU, project number PNII—IDEI code 1519/2008.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marinca, T.F., Chicinaş, I. & Isnard, O. Influence of the heat treatment conditions on the formation of CuFe2O4 from mechanical milled precursors oxides. J Therm Anal Calorim 110, 301–307 (2012). https://doi.org/10.1007/s10973-012-2250-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-012-2250-5