Abstract
NaOH/urea aqueous solution is a novel, green solvent for cellulose. To explain why cellulose just be dissolved in this solvent under −13 °C, we studied and discussed the dissolving process of cellobiose in water, urea solution, NaOH solution and NaOH/urea aqueous solution. Dissolving cellobiose in water and the urea solution absorb heat, which is an entropy-driven process. Dissolving cellobiose in NaOH solution and mixed NaOH/urea solution is exothermic, which is an enthalpy-driven process. OH− plays an important role in the dissolving process by forming a hydrogen-bonding complex. From the thermodynamic point of view, negative entropy can well interpret why cellulose must be dissolved in cold NaOH/urea aqueous solution.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A new solvent of cellulose, 7 wt% NaOH and 12 wt% urea aqueous solution with cooling, has been developed by Zhang’s group [1–7]. It is interesting that cellulose can be rapidly dissolved in this solvent system at −13 °C, but at the room temperature, the same solvent cannot dissolve the cellulose. This interesting result declared the birth of the novel, green solvent for cellulose. Therefore, to explore, the dissolving mechanism is very meaningful for the comprehensive utilization of cellulose.
There exists hydrogen bond network structure in water. In alkali solution, the network structure is destroyed by the addition of metal ions, since water molecules form stable hydrated ions with metal ions. For the OH− ions, the traditional proton transfer mechanism of OH− was questioned, recent research re-examined the water structure, the OH−–water interactions, and the mechanism of proton transfer using different methods [8–18]. Experiments and molecular modeling showed that NaOH destroyed the tetrahedral hydrogen bond network structure of the water, and there were a variety of OH−–H2O complex (H7O4 −, H9O5 −, H11O6 −) in NaOH aqueous solution [18]. The distribution of these complexes depended on the counter-ion (Na +) concentration [14, 18].
For urea aqueous solutions, Frank and Franks [19] proposed that urea destroys the hydrogen bond network structure of the water through the indirect effects of the thermodynamic behavior in aqueous solution, which is also known as F & F model. They proposed that the urea is the destroyer of the water structure, and weakening the role of the hydrogen-bonding structure of water causes the damage. This view successfully explained why hydrocarbon solubility increases [20] in urea aqueous solution. The NMR [21], X-ray [22], Raman [23], IR [24] and other experimental results also confirmed this view. In these experimental techniques, IR is sensitive to detect the hydrogen bonds. So, IR has been used to explore the aqueous solution of urea and urea crystals for the structural information. However, there still exists the difficulty to interpret the spectra.
Schellman, Kreschek, Scheraga, [25] and Stokes [26] summarized the physical and chemical properties of aqueous urea as follows: urea molecules formed the dimer or oligomer clusters through hydrogen bonds, and urea clusters played a dominant role in the dissolution of the solute, which was known as SKSS model. This model of urea aqueous solution was supported by molecular dynamics, but in the computational chemistry, the model depends on the water dimer potential function [27, 28]. The osmotic pressure measurements found that urea can form dimer and bigger cluster structure [29]. While Keuleers etc. [24] and Lee etc. [30] showed that there do not exist any hydrogen bonds between urea and urea.
For the NaOH/urea aqueous mixture solution, using the DSC and viscosity techniques, Jie Cai et al. [31] showed that in NaOH and urea aqueous solution, there exist urea hydrate and NaOH hydrate. However, In NaOH/urea aqueous solution, NaOH hydrate structure is more stable, and urea hydrate structure can be destroyed by OH−, since OH− can easily formed a new hydrogen bond network structure with the NaOH hydrate and free water. When 7 wt% NaOH/12 wt% urea aqueous solution is cooled to low temperatures, hydrogen bonds among NaOH hydrate, urea hydrate and water clusters increase, and the hydrogen bond network form a larger complex structure.
To explain why cellulose can be dissolved in the NaOH/urea aqueous solution at low temperature, we should study the interaction between the solvent and cellulose. However, given the complexity of the structure of polymers, to study the interaction between the solvent and d-cellobiose, which is the monomer of cellulose, is necessary. To understand the dissolving process, calorimeter is a powerful technique [32, 33]. In this paper, we measured the solution enthalpy of d-cellobiose in different solvent systems to better understand the structure of these solvent systems and the dissolution mechanism. In this paper, the solvent system includes distilled water, sodium hydroxide solution, urea solution and NaOH/urea aqueous solution.
Experimental
Experimental measurement was carried out at isoperibol solution calorimeter made by us [34]. The calorimeter is a 100-mL thermos glass liner vessel fitted with a thermistor for sensing temperature, and a heater for calibration and equilibration and a reaction tanks stirrer with speed of 375 r/min. This equipment has a resolution in temperature controlling system of 0.001 K, and in temperature measurement of 5 × 10−5 K.
The calorimeter was tested for the solution process of KCl in double-distilled water. We added the precisely weighted KCl (about 0.06 g) into the 100.00 mL distilled water and measured the solution enthalpy at 298.15 K. The results showed good agreement with literature data (17.57 ± 0.14 vs 17.55 ± 0.02 kJ/mol [35]).
All reagents were of analytical pure (min. 99%) and were used with further drying. The cellobiose to be dissolved was weighted on a Satorius BS 124S analytical balance with a precision of ±0.1 mg. In each experiment, about 0.04 g cellobiose was added into 100.00 mL solutions. Each enthalpy value resulted from an average of at least three individual experiments, with a relative standard deviation always less than 3%.
Results and discussion
The solution enthalpies of cellobiose in water and urea solution
Table 1 and Fig. 1 summarize the solution enthalpies (Δsol H) of cellobiose in water and urea solution with different concentrations at 303.15 K. (The concentration is expressed as molar ratio \( n_{\text{Ur}}:n_{{{\text{H}}{}_{ 2}{\text{O}}}} \)).
Table 1 and Fig. 1 show that Cellobiose dissolving in water showed significant endothermic effect, and dissolving in urea solution absorbs even more heat than in water and the absorbing heat increased with the increasing urea concentration. The endothermic effect of Cellobiose dissolved in water has related to the damage of the hydrogen bond network structure of water. Liquid water exists in orderly tetrahedral hydrogen bond network structure. When the cellobiose is dissolved in water, its hydroxyl groups form hydrogen bonds with water. At the same time, the forming of hydrogen bonds lead to the breaking of corresponding more hydrogen bonds in water’s network structures, so the overall effect show an endothermic effect. Since ordered hydrogen bond network structure is broken and even collapse, which means chaos increasing, we can draw a conclusion that the solution entropy of cellobiose in water is positive. From the thermodynamic point of view, as an endothermic dissolution process, if the dissolution behavior can occur spontaneously, it is necessary to have positive dissolution entropy. Therefore, cellobiose dissolving in water is a typical entropy-driven process.
When the urea is added into the water, urea will rebuild the hydrogen bond network structure because of the structural similarity between urea and water. However, the volume of urea molecules is bigger than that of water, and urea can form a hydrogen bond network from multiple directions structure, so the hydrogen bond network structure of the urea–water mixture is unstable than that of pure water. Therefore, when cellobiose dissolved in urea solution, the urea solution’s hydrogen bond network is more easily been damaged, which showed a larger endothermic effect.
The solution enthalpies of fructose and glucose were measured in water and urea solution, which are larger than of cellobiose. The explanation relate to the spatial structure difference between the monosaccharide and cellobiose (see Table 2). Comparing with cellobiose, hydroxyl groups of glucose and fructose can fully form hydrogen bonds structure with the solvent, because its hydroxyl groups have little steric hindrance. So monosaccharide form more hydrogen bonds in urea solution, and more likely to destroy the solvent hydrogen bonds.
We also measured the solution enthalpies under different temperatures. The results of cellobiose dissolved in water and urea solution were shown in Table 3.
Table 3 shows that with the temperature increasing, the solution enthalpy of cellobiose dissolving in water and urea solution decreases. The results can be interpreted as: with the temperature lowering, thermal motion of water molecules slows down and the hydrogen bond network structure will be more complete. So, cellobiose dissolving in solution under lower temperature will cause more serious damage effects of the hydrogen-bonding network. Then, the heat absorption is rising with the temperature lowering.
The solution enthalpies of cellobiose in alkali solution
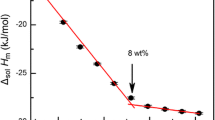
Table 4 and Fig. 2 summarize the solution enthalpies (Δsol H) of cellobiose in NaOH solution with different concentrations at 303.15 K. (The concentration is expressed as molar ratio \( n_{\text{NaOH}}:n_{{{\text{H}}_{ 2} {\text{O}}}} \)).
Table 4 and Fig. 2 shows: cellobiose dissolving in NaOH solution of low concentrations is endothermic, and cellobiose dissolving in NaOH solution of high concentration showed significant exothermic effect.
When NaOH is dissolved in water, on the one hand, the Na+ ions will form the hydrated ions with the water molecules around; on the other hand, OH− will combine some water molecules to form complexes, such as hydrate H7O4 −, H9O5 − and H11O6 − [18]. Chen et al. [14, 15] using X-ray and spectroscopy confirmed that OH− in water shows unusually high mobility. So the structure and dynamic properties of the water was changed in the presence of the OH−. In the NaOH solution of low concentration, NaOH only destroy a tiny part of the water’s tetrahedral hydrogen bond network, so the dissolving process is similar to that in water, and the solution enthalpy is positive; But in the high concentration NaOH solution, the hydrogen bond network of water are damaged, even collapse, so the damage effects of hydrogen-bonding network no longer exist. However, the OH− or hydrates of OH− can form hydrogen bonds more easily with cellobiose hydroxyl than H2O, while the OH−-cellobiose hydrogen-bonding complex is also easy to interact with the Na+ hydrate by electrostatic force. So, the overall effect of the dissolution is exothermic. The formation of the cellobiose-OH− complex indicates the negative solution entropy. From the thermodynamic point of view, cellobiose dissolving in high concentration NaOH solution is a typical enthalpy-driven process.
To discuss the role of the Na+ and OH− in the dissolving process, we also measured the solution enthalpies of cellobiose in LiOH, KOH, NaNO3, Na2SO4, NaNO3 solution. The results are shown in Table 5.
Table 5 shows: cellobiose dissolving in the different alkaline solution are all exothermic, but in the NaNO3 and Na2SO4, aqueous solution are endothermic, and the absorption of heat is almost equal to that in water, which means that as to cellobiose dissolving process in NaOH solution OH− plays a more critical role. By comparing the enthalpy of cellobiose dissolved in LiOH, NaOH and KOH solution, we can obtain a sequence that the released heat decreasing with alkali metal ion radius. Li+ is smaller and of greater charge density than Na+ and K+, so Li+ can form hydrate more easily. Also there exists stronger polar attraction between Li+ and cellobiose-OH− hydrogen-bonding complex than that of Na+ and K+. Therefore, the solution enthalpy of cellobiose dissolving in LiOH solution is greater than in the KOH and NaOH solution.
We also measured the solution enthalpies of cellobiose dissolved in NaOH solution under different temperatures. The results were shown in Table 6.
Table 6 shows that with the temperature increasing, the released heat of cellobiose dissolving in NaOH solution decreases. As the temperature decreases, hydrogen bonds between molecules become more stable. OH– or OH-hydrate will bind with cellobiose with more hydrogen bonds, so cellobiose dissolving in aqueous NaOH releases more heat.
The solution enthalpies of cellobiose in NaOH/urea aqueous solution
We measured the solution enthalpies (Δsol H) of cellobiose in NaOH/urea mixing aqueous solution with different concentrations. At 303.15 K, the results are shown in Table 7 and Fig. 3.
Table 7 and Fig. 3 show that the cellobiose dissolving process in NaOH/urea aqueous solution is exothermic. With NaOH concentration increasing, the released heat is increasing. Comparing with the results in Table 4, the solution enthalpy of cellobiose in mixed solvent is greater than that in NaOH solution.
When cellobiose is dissolved in the NaOH/urea aqueous solution, cellobiose hydroxyl proton will form the hydrogen bonds more easily with OH− or OH− hydrate, then OH− combine to the polar oxygen atoms of carbonyl of urea molecule to form hydrogen bonds through the rapid exchanging of proton. And Na+ or Na+ hydrates are also participating in this process through the electrostatic interaction. Nitrogen atoms of urea are also involved in other hydrogen bonds; the bonding process is repeated throughout the system by the pattern. Therefore, the dissolution process of cellobiose in mixed solution will form a large hydrogen-bonding complex concerned with cellobiose, OH−, Na+ and urea. The formation of the hydrogen-bonding complex plays an important role in the dissolving process, and it can interpret why the solution enthalpy of cellobiose in urea/NaOH aqueous solution is greater than in the NaOH solution. Also the formation of the complex indicates that the dissolving process has negative solution entropy of big value. So we can conclude that the dissolving process is a typical enthalpy-driven process of negative enthalpy and negative entropy. From the thermodynamic point of view, negative entropy can well interpret why cellulose must be dissolved in cold NaOH/urea aqueous solution.
There is an interesting result in this experiment. While the dissolving of cellobiose in urea solution is endothermic, and dissolving in NaOH solution of low concentration (\( n_{\text{NaOH}}:n_{{{\text{H}}_{ 2} {\text{O}}}} \) = 0.0056:1) is endothermic, but dissolving in the mixed NaOH/urea aqueous solution (\( n_{\text{NaOH}}:n_{\text{Ur}}:n_{{{\text{H}}_{ 2} {\text{O}}}} \) = 0.0056:0.0444:1) is exothermic. The reason is that the hydrogen bond network structure in urea solution is unstable than that in water. So, the adding of NaOH with even low concentration can easily destroy the hydrogen bond network in urea solution.
We also measured the solution enthalpies under different temperatures. The results of cellobiose dissolved in NaOH/urea aqueous solution with concentration \( n_{\text{NaOH}}:n_{\text{Ur}}:n_{{{\text{H}}_{ 2} {\text{O}}}} \) = 0.0389:0.0444:1 were shown in Table 8. Table 8 shows that with the temperature increasing, the released heat of cellobiose dissolving in mixed solution decreases. The reason is same as Part 3.2.
Jie Cai [31] has studied the NaOH/urea aqueous solution by the DSC. The results show that OH− makes proton of amino exchange between water molecules and urea. NaOH destroy the hydrogen bonding of urea hydrate structure and form stronger hydrogen bonds with the water molecules, which consistent with Roy’s [36] results. So, this experiment can prove that even a small amount of NaOH adding into urea solution can have a huge impact on the structure, which fully indicate the important role of OH−.
Conclusions
In this paper, we studied and discussed the dissolving process of cellobiose in water, urea solution, NaOH solution and NaOH/urea solution. Dissolving cellobiose in water and the urea solution absorb heat, and it is an entropy-driven process. The results relate to the damage effect of the hydrogen-bonding structure in water and urea solution. Dissolving cellobiose in NaOH solution of high concentration is exothermic. This process is an enthalpy-driven one. OH− plays an important role in the dissolving process by forming a cellobiose-OH− hydrogen-bonding complex. Finally, cellobiose dissolving in NaOH/urea aqueous solution is exothermic with a greater value than in NaOH solution. It is also an enthalpy-driven process. From the thermodynamic point of view, negative entropy can well interpret why cellulose must be dissolved in cold NaOH/urea aqueous solution.
References
Cai J, Zhang L, Liu S, Liu Y, Xu X, Chen X, Chu B, Guo X, Xu J, Cheng H, Han CC, Kuga S. Dynamic self-assembly induced rapid dissolution of cellulose at low temperatures. Macromolecules. 2008;41:9345–51.
Cai J, Zhang L. Unique gelation behavior of cellulose in NaOH/urea aqueous solution. Biomacromolecules. 2006;7:183–9.
Cai J, Zhang L, Zhou J, Qi H, Chen H, Kondo T, Chen X, Chu B. Multifilament fibers based on dissolution of cellulose in NaOH/urea aqueous solution: structure and properties. Adv Mater. 2007;19:821–5.
Qi H, Chang C, Zhang L. Properties and applications of biodegradable transparent and photoluminescent cellulose films prepared via a green process. Green Chem. 2009;11:177–84.
Luo X, Liu S, Zhou J, Zhang L. Properties of films composed of cellulose nanowhiskers and a cellulose matrix regenerated from alkali/urea solution. J Mater Chem. 2009;19:3538–45.
Chang C, Peng J, Zhang L, Pang D. Strongly fluorescent hydrogels with quantum dots embedded in cellulose matrices. J Mater Chem. 2009;19:7771–6.
Liu S, Zhang L, Zhou J, Xiang J, Sun J, Guan J. Fiberlike Fe2O3 macroporous nanomaterials fabricated by calcinating regenerate cellulose composite fibers. Chem Mater. 2008;20:3623–8.
Botti A, Bruni F, Imberti S, Ricci MA, Soper AK. Ions in water: the microscopic structure of concentrated NaOH solutions. J Chem Phys. 2004;120:10154–62.
Botti A, Bruni F, Imberti S, Ricci MA, Soper AK. Solvation shell of OH− ions in water. J Mol Liq. 2005;117:81–4.
Woutersen S, Emmerichs U, Bakker HJ. Femtosecond Mid-IR pump-probe spectroscopy of liquid water: evidence for a two-component structure. Science. 1997;278:658–60.
Bakker HJ, Nienhuys HK. Delocalization of protons in liquid water. Science. 2002;297:587–90.
Nienhuys HK, Lock AJ, Santen RA, Bakker HJ. Dynamics of water molecules in an alkaline environment. J Chem Phys. 2002;117:8021–9.
Omta AW, Kropman MF, Woutersen S, Bakker HJ. Negligible effect of ions on the hydrogen-bond structure in liquid water. Science. 2003;301:347–9.
Chen B, Ivanov I, Park JM, Parrinello M, Klein ML. Solvation structure and mobility mechanism of OH−: a Car–Parrinello molecular dynamics investigation of alkaline solutions. J Phys Chem B. 2002;106:12006–16.
Chen B, Park JM, Ivanov I, Tabacchi G, Klein ML, Parrinello M. First-principles study of aqueous hydroxide solutions. J Am Chem Soc. 2002;124:8534–5.
Tuckerman ME, Marx D, Parrinello M. The nature and transport mechanism of hydrated hydroxide ions in aqueous solution. Nature. 2002;417:925–9.
Ludwig R. New insight into the transport mechanism of hydrated hydroxide ions in water. Angew Chem Int Ed. 2003;42:258–60.
Ludwig R. Water: from clusters to the bulk. Angew Chem Int Ed. 2001;40:1808–27.
Frank HS, Franks F. Structural approach to the solvent power of water for hydrocarbons; urea as a structure breaker. J Chem Phys. 1968;48:4746.
Wetlaufer DB, Malik SK, Stoller L, Coffin RI. Nonpolar group participation in the denaturation of proteins by urea and guanidinium salts. Model compound studies. J Am Chem Soc. 1964;86:508–14.
Finer EG, Franks F, Tait MJ. Nuclear magnetic resonance studies of aqueous urea solutions. J Am Chem Soc. 1972;94:4424–9.
Adams R, Balyuzi HHM, Burge RE. X-ray diffraction studies of aqueous solutions of urea. Appl Crystallogr. 1977;10:256–8.
Hoccart X, Turrel G. Raman spectroscopic investigation of the dynamics of urea–water complexes. J Chem Phys. 1993;99:8498–503.
Keuleers R, Rousseau B, Alsenoy CV, Desseyn HO. Vibrational analysis of urea. J Phys Chem A. 1999;103:4621–30.
Kresheck GC, Scheraga HA. The temperature dependence of the enthalpy of formation of the amide hydrogen bond: the urea model. J Phys Chem. 1965;69:1704–6.
Stokes RH. Thermodynamics of aqueous urea solutions. Aust J Chem. 1967;20:2087–100.
Kuharski RA, Rossky PJ. Molecular dynamics study of solvation in urea water solution. J Am Chem Soc. 1984;106:5786–93.
Kuharski RA, Rossky PJ. Solvation of hydrophobic species in aqueous urea solution: a molecular dynamics study. J Am Chem Soc. 1984;106:5794–800.
Jakli G, van Hook WA. Isotope effects in aqueous systems. 12. Thermodynamics of urea-h4/water and urea-d4/water-d2 solutions. J Phys Chem. 1981;85:3480–93.
Lee C, Stahlberg EA, Fitzgerald G. Chemical structure of urea in water. J Phys Chem. 1995;99:17737–41.
Cai J. Dissolution of cellulose in alkali hydroxide/urea aqueous systems, structure and properties of new materials based on them. Doctor degree thesis, Wuhan University. 2006.
Piekarski H, Nowicka B. Calorimetric studies of interactions of some peptides with electrolytes, urea and ethanol in water at 298.15 K. J Therm Anal Cal. 2010;102:31–6.
Cooper A. Microcalorimetry of heat capacity and volumetric changes in biomolecular interactions—The link to solvation? J Therm Anal Cal. 2011;104:69–73.
Wang CX, Song ZH, Xiong WG, Qu SS. Development of an isoperibol reaction calorimeter. Acta Phys Chim Sinica. 1991;7:586–8.
Cox JD. Recommended reference materials for the realization of physicochemical properties. Pure Appl Chem. 1974;40:399.
Roy C, Budtova T, Navard P, Bedue O. Structure of cellulose-soda solutions at low temperatures. Biomacromolecules. 2001;2:687–93.
Acknowledgements
This study was supported by the National Basic Research Program of China (973 Program, 2010CB732203).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhao, X., Chen, Y., Jiang, X. et al. The thermodynamics study on the dissolution mechanism of cellobiose in NaOH/urea aqueous solution. J Therm Anal Calorim 111, 891–896 (2013). https://doi.org/10.1007/s10973-012-2217-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-012-2217-6