Abstract
Silicate–phosphate glasses of the XYPO4–SiO2 and XYPO4–SiO2–AlPO4 (where X = Na+ and/or K+ and Y = Ca2+ and/or Mg2+) systems have been the subject of the presented investigations. Bioactive glasses from these systems are the base for obtaining glass-crystalline biomaterials through a direct crystallization. However, growth of crystalline phases very adversely affects the bioactivity of the glasses. Uncontrolled growth of crystalline phases can be reduced by means of a glass phase separation phenomenon in the silicate–phosphate glasses because boundaries of inclusion-matrix phase may be a barrier limiting the growth of crystalline phases. Microscopic and EDX investigations which have been carried out have shown that glass phase separation occurs in glasses belonging to XYPO4–SiO2 and XYPO4–SiO2–AlPO4 systems. Introduction of aluminum ions into the glass structure leads to a rapid homogenization of its texture. Based on DSC examinations it has been found out that crystallization of the glasses of XYPO4–SiO2 systems is a multistep process. The presence of several (the number depends on the type of modifiers and glass-forming ions) clearly separated exothermic peaks in DSC curves of investigated glasses makes it possible to crystallize only the inclusions with the matrix remaining amorphous or vice versa. It has been shown that, crystallization of glasses of XYPO4–SiO2–AlPO4 system is single-stage process, which is the consequence of the homogenizing effect of aluminum ions on their texture.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Glasses of NaCaPO4–SiO2 system belong to a group of the so-called bioactive glasses, capable of forming bonds with tissue [1–7]. Application of glasses as biomaterials allows us to take the advantages of specific properties of glassy state, i.e., easy to obtain practically any shape, easy to control properties via the appropriate selection of the chemical composition, possibility to use various preparation methods as well as isotropic properties. An ideal biomaterial should be both, biochemically and biomechanically, compatible with tissue [8]. Biochemical compatibility is achieved by proper selection of chemical composition of glass. Unfortunately, the most characteristic feature is the fragility of glass, which greatly limits their application. The problem of unsatisfactory mechanical strength of bioactive glasses is not new, and there is constant search for new ways to reach the objective of eliminating (or at least reduce) the evident disadvantages of these materials. This can be achieved through the introduction of appropriate ions—mostly Al3+. The presence of Al3+ significantly improves both the mechanical strength and chemical resistance of glass. The possibility of introduction of aluminum ions into the structure of bioactive glass is very limited because of its carcinogenicity, and adverse impact on the bioactivity of the glass [9, 10]. Another way to improve the mechanical properties of bioactive glass is to carry out its partial devitrification (directed crystallization) to obtain the glass-crystalline biomaterial. In relation to the starting glasses, glass-crystalline materials are characterized by much higher mechanical strength resulting from the presence of very fine crystals that are statistically distributed in the remaining glass. The presence of randomly distributed crystals causes the properties of glass-crystalline materials to be independent from the direction of research—isotropy [11, 12]. Thus, the glass-crystalline materials allow us to combine the advantages of glassy and crystalline state. Therefore, bioactive glassy-crystalline materials exhibit significantly better mechanical and biological properties relative to the starting glasses [13, 14].
Lowering of bioactivity degree due to crystallization is the main problem in the preparation of glassy-crystalline materials [15]. In the extreme case, crystallization can convert a bioactive material into an inert one [16]. Hence, it is necessary to design the controlled glass crystallization process properly to obtain the material of predetermined texture.
To achieve the above aim detailed information about precursor glass of glass-crystalline material is required. Structural research of silicate and silicate–phosphate glasses, which have been conducted in our team for many years, allowed us to determine that they are characterized with domain structure, and its size is exceeding single tetrahedron—middle range arrangement [e.g., [17–21]]. Knowledge of domains’ structure has essential meaning for directed crystallisation of glass since devitrification of glass is based on reorientation of domains during annealing [22]. Domains can be treated as crystallization nuclei, which will decide of type of crystallizing phase [23]. Heterogeneity of glasses may also occur macroscopically as glass phase separation phenomenon. The glass phase separation in silicate–phosphate glasses of NaCaPO4–SiO2 system is revealed through occurrence of two glassy phases—spherical inclusions distributed in matrix [19, 24, 25]. Detailed spectroscopic and microscopic studies of glasses of NaCaPO4–SiO2 system with various additives (K2O, MgO, and Al2O3) allowed us to specify the influence of each ion for their structure and texture. Especially interesting seems the influence of aluminium ions on chemical composition of matrix and inclusion. Even a small addition of Al3+ (5% AlPO4) leads to inversion of chemical composition of matrix and inclusion [19, 26]. It has been shown that the exchange of alkaline ions (Na+ for K+) or alkaline earth ions (Ca2+ for Mg2+) has impact on boundary at which occurs the inversion of chemical composition of both the matrix and inclusion. In glasses of NaCaPO4–SiO2–AlPO4 and (Na,K)CaPO4–SiO2–AlPO4 systems, it happens after exceeding 25% NaCaCaPO4 or (Na,K)CaPO4; however, in glasses of KCaPO4–SiO2–AlPO4, NaMgPO4–SiO2–AlPO4, and Na(Ca,Mg)PO4–SiO2–AlPO4 systems, it occurs after exceeding 35%, respectively, in glasses of KCaPO4, NaMgPO4, and Na(Ca,Mg)PO4 [19, 26–28].
Knowledge of structure and texture is necessary during planning of directed crystallization of glasses. Of course one must also know the thermal properties of glass. The thermal properties of phosphate silicate glasses with different additives were analyzed by Stoch [29] and Wacławska and Szumera [30–32]. Thermal activity of silicate–phosphate glasses modified by the addition of modifiers in the form of CaO, MgO, Fe2O3, and MnO2 were explained on the basis of crystallochemical factors connected with the strength of the chemical bonds between the oxygen atoms and framework-forming components and modifiers. Authors according to the conception of multistage crystallization suggest that the oxygen bridges formed as a result of introducing into the silicate–phosphate structure modifiers are characterized by some strengths of bonds with oxygen atoms. The smaller the difference in the ionicity of the analyzed bonds, the lower is the strength of the oxygen bridges, and hence the easier the breaking of the bonds between them as a result of crystallization process. Therefore, in the crystallization process, there participates that part of components of the glass structure bonds of which can be broken most easily.
The glass phase separation can be beneficial for obtaining glass-crystalline materials because it gives potential abilities to reduce the problem of uncontrolled growth of crystalline phases during devitrification. Phase boundaries between inclusions and matrix can be a barrier, limiting expansion of crystals during devitrification.
Experimental
To conduct the research, glasses of NaCaCaPO4 system with different ions (K+, Mg2+, and Al3+) with compositions included in Table 1 were chosen. In the case of glasses containing aluminium ions, glasses in which the earlier mentioned inversion of chemical composition already occurred were chosen. Such selection causes that compositions of matrices and inclusions of glasses containing Al3+ ions and those not containing them are similar.
The standard sol–gel method was selected to obtain the materials of the highest possible homogeneity. Tetraethylorthosilicate—TEOS—(SiO2), Ca(NO3)2·4H2O (CaO), Na3PO4·12H2O (Na2O), K2HPO4 (K2O) Al(NO3)3·3H2O (Al2O3), Mg(NO3)2·6H2O (MgO), and H3PO4 (P2O5) were used to introduce particular oxides. All the chemicals used have been of analytic grade. The obtained gels were dried at room temperature (30 days) and then at the temperature of 60 °C. To obtain the glassy samples, the gels were melted in platinum crucible at the temperature 1730 °C and rapidly cooled on the cast iron plate. The gels were heated at a heating rate of 5 °C min−1 till 1730 °C and then stabilized in the same temperature for 2 h.
Annealing of glasses at temperatures determined on the basis of thermal research was carried out in a gradient furnace.
X-ray measurement of all the samples were carried out using FPM Seifert XRD 7 with a step of 0.01 deg and a collection time of 5 s.
FIR (far infrared) spectroscopic measurements of the glasses were made using a Bio-Rad FTS 60V spectrometer. Transmission technique—samples as polyethylene pellets. Spectra were collected after 2000 scans at 4 cm−1 resolution.
EDX spectra were measured on a JEOL 5400 scanning microscope with a microprobe analyzer LINK ISIS (Oxford Instrument).
The thermal stabilities of the obtained glasses were determined by DSC measurements conducted on STA 449 F3 Jupiter®7 (Netzsch) operating in the heat flux DSC mode.
The samples were heated in platinum crucibles at a heating rate of 10 °C min−1 up to 1100 °C in dry nitrogen atmosphere.
Results
Knowledge of microstructure and structure of glass gives the basis to plan the process of directed crystallization to obtain glass-crystalline biomaterials. To plan directed crystallization of glass it is necessary to define characteristic temperatures for glass state, especially the so called devitrification temperature (TD), in which occurs rapid glass crystallization. Main aim of this study was to define the impact of modifiers (K+, Ca2+, and Mg2+) and glass-forming ions (Al3+) on crystallization of silica phosphate glasses of NaCaPO4–SiO2 system.
Amorphous nature of the obtained materials has been confirmed by XRD and FIR studies. XRD patterns of all the samples contain exclusively the raised background typical for amorphous materials. In the FIR spectra, no intensive bands can be seen which unequivocally shows that the long-range order in the materials is lacking. All the obtained glasses were nontransparent, with their confirmed amorphous nature indicating the occurrence of glass phase separation. Research conducted on scanning microscope (Figs. 1, 2) clearly indicates the occurrence of glass phase separation phenomenon—spherical inclusions in matrix. Owing to the confirmed (XRD and FIR) amorphousness of samples, it can clearly be stated that both matrix and inclusions are amorphous. EDX studies (Figs. 3, 4, 5, 6) showed that inclusions and matrix differ significantly with chemical composition and hence characteristic of local arrangement. This difference causes that inclusions and matrix must have different devitrification temperatures. Therefore in chosen glasses, multistage devitrification is to be expected. Such a course of devitrification was found in glasses of NaCaPO4–SiO2 system. Conducted DTA studies allowed us to state that devitrification of glasses of NaCaPO4–SiO2 system runs in two stages. Two separated peaks on DTA curves were found at 826 and 892 °C, meaning crystallization of two different phases—separately crystallizes matrix and inclusions [23]. Addition of aluminum ions into structure of glasses causes one-stage devitrification—the presence of one peak on DTA curve at 980 °C. This effect was linked with homogenizer effect of aluminum ions on structure and microstructure of silicate–phosphate glasses [24].
Figure 7 shows the results of DSC studies for 3NaK and 3K glasses. Analysis of these curves allows us to state that, similar to glasses of NaCaPO4–SiO2 system, crystallization of 3NaK and 3K glasses runs in two stages. Layout of peaks on DSC curves of glasses of NaCaPO4–SiO2 [23] and KCaPO4–SiO2 systems is very similar. Two clear, separated peaks at 905 and 955 °C on DSC curve for 3NaK glass and 1033 and 1058 °C for 3K glass, allow us to state that, in both cases, two different phases crystallize. The presence of glass phase separation previously found allows us to believe that matrix and inclusions crystallize separately. Significant shift of peaks on DSC curve to higher temperatures is characteristic—respectively, by 80 °C for 3NaK glass and 200 °C for 3K. It is possible to state that replacement of sodium ions with potassium ions significantly improves thermal resistance of silicate–phosphate glasses of NaCaPO4–SiO2 system.
The XRD studies carried out have shown that, during crystallization of these glasses, phosphate phase crystallized as the first one and as the next one, the silicate phase—low cristobalite (Table 2). Thus, XRD studies confirm the two-stage crystallization process of 3NaK and 3K glasses.
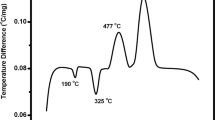
In Fig. 8, DSC curves for 3AlNaK and 4AlK glasses are shown. As mentioned earlier, glasses including aluminium ions chosen for research are those in which inversion of chemical composition of matrix and inclusion have already occurred [27], meaning that chemical composition of inclusion and matrix in given glasses (3AlNaK and 4AlK) is similar to the composition of matrix and inclusion of glasses, not containing aluminium ions (3NaK and 3K)—Figs. 3 and 4. In DSC curves for each 3AlNaK and 4AlK, only one clear peak is visible at 846 °C (3AlNaK) and 956 °C (4AlK). It is fair to say that the introduction of aluminium ions into glasses containing potassium ions decreases their thermal resistance. The presence of only one peak is a result of homogenizating influence of aluminium ions on texture and structure of given glasses [27]. Homogenization shows through occurrence of seemingly lesser amount of spherical inclusions (Fig. 1). Small amount of inclusions causes that effect of their crystallization is almost unseen. More detailed analysis of DSC curves allows us to distinguish small exothermal effects connected to crystallization of inclusions—at ~930 °C (3AlNaK) and ~1000 °C (4AlK). XRD studies show (Table 2) that during the crystallization of these glasses only one phase crystallized—phosphate phase.
Interpretation of results from thermal studies on glasses in which calcium ions were replaced with magnesium ions is much harder—Fig. 9 (Na(Ca,Mg)PO4–SiO2 and NaMgPO4–SiO2 systems). Replacement of half of Ca2+ ions for Mg2 causes reduction in thermal resistance of glass—crystallization starts at 699 °C. Full replacement leads to insignificant rise of thermal resistance of glass (comparing to glass of NaCaPO4–SiO2 system)—crystallization starts at 845 °C. On both curves, there are four clearly visible exothermal effects, provided that probably four different phases crystallized. XRD studies of 3Mg and 3CaMg glasses after devitrification process allowed us to show that only three phases crystallize (Table 2). Again, the first phase to crystallize is the phosphate one and the next one is silicate phase. It is linked with heterogeneity of both texture of glass (Fig. 2) as well as structure of inclusion and matrix—domains of different characteristic occur [33]. The glass phase separation is heterogeneous, and another glass phase separation was observed in inclusions and matrix which can explain the occurrence of multi-exothermal effects. Owing to size of these inclusions, it is impossible to determine their chemical compositions using scanning microscope with EDX attachment [33].
Introduction of aluminium ions into glasses containing magnesium ions causes crystallization to run in one stage (Fig. 10). On DSC curves, peaks are visible at 711 °C (4AlMgCa) and 752 °C (4AlMg), which are connected with crystallization of matrix’. Hence, the simultaneous presence of aluminium and magnesium ions leads to reduction of thermal resistance of glasses. Similar to previous cases, due to homogenizing influence of aluminium ions on texture of silicate–phosphate glasses, the peak of inclusion crystallization is almost unseen—only small exothermal effects are visible respectively at 950 and 945 °C. XRD studies have shown that mainly phosphate phase crystallizes. A small quantity of forsterite was also fund.
Conclusions
The conducted thermal research of silicate–phosphate glasses of NaCaPO4–SiO2 system with various modifiers (K+, Ca2+, and Mg2+) and glass-forming ions (Al3+) allowed us to state that
-
1.
Homogenizing influence of aluminium ions on texture and structure of silicate–phosphate glasses causes that independent of the type of modifiers, crystallization of such glasses run in one stage. The amount of inclusions in glasses containing aluminium ions is too low to register clear exothermal peaks connected with crystallization. On DSC curves are visible only slight exothermal effects which can be linked to crystallization of inclusions.
-
2.
The glass phase separation in silicate–phosphate glasses not containing aluminium ions, independent of modifiers are characterized with multi-stage crystallization—separately crystallizes matrix and inclusions.
-
3.
Partial or whole replacement of sodium ions with potassium ions leads to significant increase in thermal resistance of silicate–phosphate glasses not containing aluminium ions. The simultaneous presence of aluminium ions decreases thermal resistance.
-
4.
Partial or whole replacement of calcium ions with potassium ions leads to decrease of thermal resistance of given glasses regardless of the presence of aluminium ions in them.
-
5.
Complicated crystallization of given silicate–phosphate glasses (Na(Ca,Mg)PO4–SiO2 and NaMgPO4–SiO2 systems) derives probably from heterogeneity of their texture and structure.
References
Hench LL. Bioceramics: from Concept to Clinic. J Am Ceram Soc. 1991;74:1487–510.
Rawlings RD. Bioactive glasses and glass-ceramics. Clin Mater. 1993;14:155–79.
Li P, Kangasniemi I, Groot K, Kokubo TJ, Yli-Urpo AU. Apatite crystallization from metastable calcium phosphate solution on sol–gel-prepared silica. J Non-Cryst Solids. 1994;168:281–6.
Hench LL, Splinter RJ, Greenlee TK, Allen WC. Bonding mechanism at the interface of ceramic prosthetic materiale. J Biomed Res Symp. 1971;2:117–41.
Filgueiras MR, La Torre G, Hench LL. Solution effects on the surface reactions of a bioactive glass. J Biomed Mater Res. 1993;27:445–53.
Hench LL, Paschall HA. Direct chemical bond of bioactive glass ceramic materials to bone and muszle. J Biom Res Symp. 1973;4:25–42.
Piotrowski G, Hench LL, Allen WC. Mechanical studies of the bone bioglass interfacial bond. J Biom Res. 1975;9:47–61.
Hench LL, Ethridge EC. Biomaterials: an interfacial approach. New York: Academic Press; 1982.
Gross U, Strunz V. The interface of various glasses and glass ceramics with a bony implantation bed. J Biomed Res. 1985;19:251–71.
Andersson OH, Karlsson KH, Kagasniemi K, Yli-Urpo A. Models for physical properties and bioactivity of phosphate opal glasses. Glasstech Ber. 1988;61:300–5.
Stand Z. Glass-ceramic materials. Amsterdam: Elsevier; 1986.
Holand W, Beall G. Glass-ceramic technology. Westerville: The American Ceramic Society; 2002.
Cao W, Hench LL. Bioactive materials. Ceram Int. 1996;22:493–507.
Bonfield W, Wang M, Tanner KE. Interfaces in analogue biomaterials. Acta Mater. 1998;46:2509–18.
Peitl O, Torre G, Hench LL. Effect of crystallization on apatite-layer formation of bioactive glass 45S5. J Biomed Mater Res. 1996;30:509–14.
Li P, Zhang F, Kokubo TJ. The effect of residual glassy phase in a bioactive glass-ceramic on the formation of its surface apatite layer in vitro. Mater Sci Mater Med. 1992;3:452–6.
Sitarz M, Handke M, Mozgawa W. Rings in the structure of silicate glasses. J Mol Struct. 1999;511–512:281–5.
Sitarz M, Handke M, Fojud Z, Jurga S. Spectroscopic studies of glassy phospho-silicate materials. J Mol Struct. 2005;744–747:621–6.
Sitarz M. Influence of modifying cations on the structure and texture of silicate–phosphate glasses. J Mol Struct. 2008;887:237–48.
Sitarz M. The structure of liquation silico-phosphate glasses. J Mol Struct. 2008;887:229–36.
Sitarz M, Fojud Z, Olejniczak Z. The aluminium effect on the structure of silico-phosphate glasses studie by NMR and FTIR. J Mol Struct. 2009;924–926:107–10.
Görlich E. Glassy State. Cracow: AGH no.155; 1989 (in Polish).
Sitarz M, Szumera M. Crystallization of silico-phosphate glasses. J Therm Anal Cal. 2008;91:255–60.
Handke M, Sitarz M, Rokita M, Galuskin EW. Vibrational spectra of phospho-silicate biomaterials. J Mol Struct. 2003;651–653:39–54.
Sitarz M, Rokita M, Handke M, Galuskin EW. Structural studies of the NaCaPO4–SiO2 sol–gel derived materials. J Mol Struct. 2003;651–653:489–98.
Sitarz M. The effect of inversion of matrix and inclusions composition in liquation phospho-silicate glasses. Spectrochim Acta Part A. 2011;79:739–42.
Sitarz M. Structure and texture of glasses belonging to KCaPO4–SiO2 and KCaPO4–SiO2–AlPO4 systems. Phys Chem Glasses. 2010;51:179–86.
Bułat K, Sitarz M, Gajewicz M. Microstructure of silico-phosphate glasses in the NaMgPO4–SiO2 system. Pol Ceram Bull. 2011;2:391–5.
Stoch L. Flexibility of structure as a criterion of glass formation and stability. Optica Applicata. 2000;30:647–55.
Wacławska I, Szumera M. Influence of MgO (CaO) on the structure of silicate–phosphate glasses TA and NMR study. J Therm Anal Cal. 2006;84:185–90.
Szumera M, Wacławska I. Thermal behaviour of Fe-doped silicate–phosphate glasses. J Therm Anal Cal. 2010;88:151–6.
Szumera M, Wacławska I. Thermal study of Mn-containing silicate–phosphate glasses. J Thermal Anal Cal. 2011. doi 10.1007/s10973-011-1941-7.
Gajewicz M. Structural studies of phospho-silicate glasses. Cracow: Master thesis; 2009 (in Polish).
Acknowledgements
This project was funded by the National Science Center awarded on the basis of the decision number DEC-2011/01/N/ST8/07425.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sitarz, M., Bulat, K. & Szumera, M. Influence of modifiers and glass-forming ions on the crystallization of glasses of the NaCaPO4–SiO2 system. J Therm Anal Calorim 109, 577–584 (2012). https://doi.org/10.1007/s10973-011-2156-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-011-2156-7