Abstract
A new semi-organic nonlinear optical material glycyl-l-alanine hydrochloride (GLAH) was grown successfully by slow evaporation solution growth method. The solubility of GLAH was estimated for a wide range of temperatures. Large size single crystal of size 15 × 9 × 6 mm3 was grown at room temperature. The grown crystal was subjected to single crystal X-ray diffraction study which confirms that the grown crystal is monoclinic in nature with the space group P21. The molecular weight of the title compound was estimated by mass spectrometry. Functional groups and the modes of vibrations were identified by FT-IR spectral analysis. The UV–visible spectral study reveals that the percentage of optical transmission of the sample is very high in the entire visible and UV regions. The second harmonic generation of the crystal was confirmed by the Kurtz and Perry technique. The hardness of the sample was tested by microhardness test which shows that the grown crystal belongs to the soft category of materials. The thermal stability of the compound was studied by TG–DTA analyses which indicate that the crystal is thermally stable up to 248.6 °C.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Recently, there is substantial interest in the synthesis of new nonlinear optical (NLO) material, both organic and inorganic with large second-order optical nonlinearities, for NLO applications in frequency conversion, optical telecommunication, image processing, optical computing, and data storage devices [1–3]. Interestingly amino acids exhibit specific features such as molecular chiralty, absence of strongly conjugated bonds and zwitterionic nature of molecules [4]. Amino acids can be used as chiral auxiliaries for nitro aromatics and other donor- acceptor molecules with large hyperpolarizabilities and a basis for synthesizing organic and inorganic compounds [5]. Numerous studies on semi-organic amino acid compounds such as l-argininium dinitrate [6], l-arginine hydrochloride [7], l-alanine acetate [8], and glycine sodium nitrate [9] have been reported. The amino acid l-alanine forms a number of complexes in coordination with inorganic acid and salts to produce outstanding materials for NLO applications. Single crystal of l-alanine with excellent NLO property was first reported by Misoguti et al. [10]. Many authors have intensively studied the growth and characterization of optically active amino acid l-alanine crystals grown by solution growth method [11, 12]. Motivated by the previous work, the team has successfully grown good optical quality single crystals of glycyl l-alanine hydrochloride (GLAH) by slow evaporation solution growth method. Spectral, optical, and thermal characterizations are important techniques for material characterization. Hence, several authors have followed these techniques for material characterization [13–22]. In this work, the grown compound GLAH was analyzed by single crystal X-ray diffraction (XRD), mass spectral analysis, Fourier transform infrared (FT-IR), UV–visible, microhardness, and thermal studies.
Experimental
Synthesis
The stoichiometric amount of l-alanine (AR grade from E-Merck India Ltd.), l-glycine (Analar grade) and excess of hydrochloric acid were taken with 50 ml of Millipore water to synthesize glycyl-l-alanine hydrochloride (GLAH) with the molecular formula C6N2O3H11Cl. The synthesized material was then recrystallized from water and dried.
Solubility
The recrystallized salt was used for solubility studies. The solubility of GLAH in Millipore water was determined by dissolving the solute in water taken in an air tight container maintained at a constant temperature with continuous stirring and after attaining saturation, the equilibrium concentration of the solute was analyzed gravimetrically. The solubility of the synthesized GLAH was determined in the temperature range 30–60 °C (g/100 ml H2O) in steps of 5 °C using a constant temperature bath of accuracy ±0.01 °C. The variation of solubility with temperature is shown in Fig. 1. From the curve, it is observed that the material possesses a positive gradient of solubility. Hence, Millipore water is sufficient for the growth of good quality single crystals of reasonable size.
Crystal growth
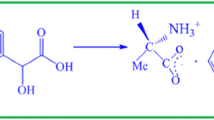
The solution was saturated at 35 °C and seed crystals were formed due to spontaneous nucleation. In this process, three drops of hydrogen peroxide were added to the mother solution of glycyl- l-alanine hydrochloride to inhibit the growth of microbes [23]. Single crystal up to a size of 15 × 9 × 6 mm3 was grown in a period of 45 days. The grown GLAH single crystal is shown in Fig. 2. The molecular structure of as grown crystal of GLAH is as shown in Fig. 3.
Results and discussion
Single crystal XRD analysis
Single crystal XRD studies of GLAH were carried out using Enraf-Nonius CAD4 single X-ray diffractometer. The single crystal XRD data of GLAH indicates that it crystallizes in monoclinic system with P21 space group. The calculated lattice parameters are a = 5.73 Å, b = 18.27 Å, c = 7.87 Å and α = γ = 90°, β = 96.72°, and V = 818 Å3. These values are in good agreement with the data reported in the literature [24].
Mass spectral analysis
Mass spectrometry is an analytical technique that measures the mass to charge ratio of charged particles. It is used for determining masses of particles, elemental composition of a sample or a molecule and for elucidating the chemical structure of molecules such as peptides and other chemical compounds. The mass spectrum of GLAH was recorded using a thermo-electron JEOL GC mate Direct Probe ion trap mass spectrometer and is shown in Fig. 4. The source voltage was 5 kV at the capillary temperature 375 °C with an ionization mode EI+. Nitrogen was used both as a sheath and auxiliary gas crystal. The mass (m) to charge (z) ratio of the sample was scanned and the experimental molecular weight (182.1552 amu) was found to be in good agreement with theoretical value (182.596 amu). The slight deviation from the proposed molecular weight of the crystalline compound can be attributed to the presence of fractional impurities in the crystal. The spectrum of GLAH showed a protonated molecular adduct ion peak at m/z = 182(M + H). [(C5N2O3H10)Cl·H+]. The loss of (CH3–CH–NH2–CO) moiety from the molecular ion (72 amu) gives the peak at m/z 92. The loss of [CH3–CH–NH2–CO·HCl]− moiety gives the peak at m/z 108. Thus, the formation of title compound was established.
FT-IR analysis
The presence of the functional groups was qualitatively analyzed by the Infra red spectrum. The FT-IR analysis was recorded in the region 400–4000 cm−1 using Bruker IFS 66V spectrometer to confirm the presence of functional groups in the grown crystal. The middle IR spectrum of GLAH is shown in Fig. 5. It is observed that the broad envelope between 2508 and 3081 cm−1 is due to overlapping of NH3 and CH stretching modes. The NH2 group is protonated by the COOH group, giving rise to NH3 + and COO− groups. In the overtone region, there is a sharp intense peak at 2112 cm−1 which is assigned to combinational and asymmetrical bending vibration of NH3 +. The bending modes of CH3 are resolved at 1362 and 1505 cm−1. The peak at 1114 cm−1 is due to C–O stretch and the O–H bend of COOH group is observed at 1236 cm−1. The lack of any strong IR band at 1700 cm−1 clearly indicates the existence of the COO− ion in zwitterionic form [25]. The symmetric and asymmetric NH3 + stretching vibrations appear at frequencies 3081 and 2938 cm−1, respectively. The C–H and N–H bending modes are observed at 1307 cm−1 and the absorption peak at 1594 cm−1 confirms the presence of NH3 bending [26]. A peptide bond CO–NH is formed between the carboxyl group COOH in l-alanine and amino group NH2 which is clearly visible in the spectrum at 1620 cm−1 [27]. The O–H symmetric stretching vibration of water is seen at 1455 cm−1 [28].
UV–visible spectral study
The absorption spectrum plays a vital role to identify a potential NLO material which has a wide transparency window without any absorption at the fundamental and second harmonic wavelength. The UV–visible spectral analysis of GLAH crystal was carried out between 200–2500 nm covering the entire near ultra violet, visible, and near infra- red regions, using a Perkin-Elmer Lambda 25 UV spectrometer. The recorded optical absorption spectrum of the compound is shown in Fig. 6. The absorbance is very less in the UV and the entire visible region, which is an interesting observation in this material. The lower cut-off wavelength is found to be 280 nm which is a desirable property for the material for NLO applications. The optical losses in the higher wavelength side could also be reduced by optimizing the growth conditions toward the production of crystals of higher quality.
Nonlinear optical study
In order to find out the NLO property of GLAH, second harmonic efficiency test was performed by the Kurtz and Perry powder technique [29] using Q-switched, mode locked Nd:YAG laser operating at the fundamental wavelength 1064 nm, generating about 2.5 mJ/pulse. This laser can be operated in two modes. In the single shot mode, the laser emits a single 8 ns pulse. In the multishot mode, the laser produces a continuous train of 8 ns laser pulses at a repetition rate of 10 Hz. In this study, the single shot mode of 8 ns laser pulses with a spot radius of 1 mm was used. The experimental set up used a mirror and 50/50 beam splitter, to generate a beam with pulse energy of 6 mJ. The input laser beam was passed through an IR reflector and then directed on the microcrystalline powdered sample packed in a capillary tube of diameter 0.154 mm. The light emitted by the sample was detected by photo diode detector and oscilloscope assembly. For the SHG efficiency measurements, microcrystalline material of KDP was used for comparison. The second harmonic generation was confirmed by the green emission of wavelength 532 nm from the crystalline sample. It was found that the efficiency of SHG is 15% of that of the standard KDP.
Microhardness study
Microhardness studies were made on the flat polished face of the grown crystal at room temperature for various loads from 5 to 50 g using HMV Shimadzu microhardness V tester, fitted with Vickers diamond indenter. Since crack initiation and material chipping became significant beyond 50 g of the applied load, hardness test could not be carried out above this load. The length of the two diagonals of the indentations were measured on the (010) and (001) planes and the Vickers microhardness number (H v) was computed using the relation
where P is the indenter load in kg and d is the diagonal length of the impression in mm. Figure 7 illustrates the Vickers hardness profile as a function of applied load. The plot suggests that GLAH crystal has a fairly high Hv value of 48 kg/mm2 for an applied load of 50 g, which indicates that the grown crystal has a high VHN value comparable with other organic and semiorganic NLO crystals. Hardness is a measure of the resistance to deformation which is directly related to the forces that exist between the atoms in the solid. In the present investigation, the hardness number of GLAH is found to increase with increase in load, confirming the hardness property of the crystal. The value of work hardening coefficient n is estimated using the Meyers relation P = ad n where a is an arbitrary constant. The work hardening coefficient of the grown crystal is found to be 2.58. As the value of n is greater than 2, the hardness of the material belongs to soft category.
Thermal studies
The thermal stability of GLAH single crystal was estimated by TG and DTA techniques. Simultaneously thermo gravimetric analysis and differential thermal analysis were carried out for the crystal using a NETZSCH STA 409C/CD thermal analyzer. A powder sample of 20.100 mg was kept in nitrogen atmosphere in the temperature range 25–1200 °C with a heating rate of 10 K/min. The crucible used was of alumina (Al2O3) which served as a reference for the sample. Simultaneously recorded TG and DTA curves are shown in Figs. 8 and 9. It is evident from TG curve that the grown crystal possesses a very good thermal stability up to 248.6 °C. There is no weight loss up to 248.6 °C and major weight loss of about 40% is observed at 250.8 °C which may be attributed to the elimination of CO2 from the crystal. However, two stages of weight loss have been observed above 250.8 °C. The first stage occurs at 289.2 °C with 30% of weight loss, which is attributed to the removal of ammonia. The second stage at 368.4 °C with 9.3% of weight loss indicates that the sample has collapsed through the breakage of peptide bond in the crystal. In the DTA trace, a sharp endothermic peak observed around 248.6 °C corresponds to the decomposition temperature of the material which coincides with the TGA trace. Another important observation is that there is no phase transition till the material melts and enhances the temperature range for the utility of the crystal for NLO applications [30].
Conclusions
Single crystals of GLAH were grown by slow evaporation technique in a period of 45 days. The XRD analysis confirms that the crystal belongs to monoclinic system with the space group P21. The experimental value of the compound from the mass spectrum is found to be in close agreement with the theoretical value. Functional groups and the modes of vibrations have been identified by FTIR spectral analysis. The minimum absorption in the entire visible range and lower cut-off wavelength near 280 nm shows the suitability of this material for NLO applications. The NLO property analysed with Kurtz Powder technique confirms the non linear nature of the crystal. GLAH crystal is found to be mechanically stable up to 50 g and the increase of the work hardening coefficient may be due to the dislocation motion. The thermal analysis reveals that the grown crystal is highly stable which ensures the suitability of the material for possible application in lasers where the crystals are required to withstand high temperatures. Preliminary studies of GLAH suggest that the crystal is a suitable material for nonlinear applications and photonic device fabrication.
References
Vijayan N, Rajasekaran S, Bhagavannarayana G, Ramesh Babu R, Gopalakrishnan R, Palanichamy M, Ramasamy P. Growth and characterization of nonlinear optical amino acid single crystal: l-alanine. Cryst Growth Des. 2006;6(11):2441–5.
Alosious Gonsago C, Helen Merina Albert, Malliga P, Joseph Arul Pragasam A. Crystallisation, spectral, and thermal characterization of l-histidine methyl ester dihydrochloride (LHMED). J Therm Anal Calorim. 2011. doi:10.1007/s10973-011-1719-y.
Alosious Gonsago C, Helen Merina Albert, Malliga P, Joseph Arul Pragasam A. Growth and characterization of pure and thiourea doped l-histidine single crystals. Mater Manuf Process. 2011. doi:10.1080/10426914.2011.585495.
Lydia Caroline M, Sankar R, Indirani RM, Vasudevan S. Growth, optical, thermal and dielectric studies of an amino acid organic nonlinear optical material: l-alanine. Mater Chem Phys. 2009;114:490–4.
Sethuraman K, Ramesh Babu R, Gopalakrishnan R, Ramasamy P. Synthesis, growth and characterisation of a new semiorganic nonlinear optical crystal: l-alanine sodium nitrate (LASN). Cryst Growth Des. 2008;8(6):1863–9.
Thomas PC, Thomas J, Pakiam Julius J, Madhavan J, Selvakumar S, Sagayaraj P. Growth and characterization of l-argininium dinitrate. J Cryst Growth. 2005;277:303–7.
Meera K, Muralidharan R, Dhanasekaran E, Prapun M, Ramasamy P. Growth of nonlinear optical material: l-arginine hydrochloride and its characterization. J Cryst Growth. 2004;263:510–6.
Mohankumar R, Rajanbabu D, Jayaraman D, Jayavel R, Kitamura K. Studies on the growth aspects of semiorganic l-alanine acetate: a promising NLO crystal. J Cryst Growth. 2005;275:e1935–9.
Narayan Bhat M, Dharmaprakash S. Growth of nonlinear optical γ-glycine crystals. J Cryst Growth. 2002;236:376–80.
Misoguti L, Varela AT, Nunes FD, Bagnato VS, Melo FEA, Mendes Filho J, Zilio SC. Optical properties of l-alanine organic crystals. Opt Mater. 1996;6:147–52.
Razzetti C, Ardoina M, Zanotti L, Zha M, Paorici C. Solution growth and characterization of l-alanine single crystals. Cryst Res Technol. 2002;37:456–65.
Vijayan N, Rajasekaran S, Bhagavannarayana G, Ramesh Babu R, Gopalakrishnan R, Palanichamy M, Ramasamy P. Growth and characterization of nonlinear optical amino acid single crystal: l-alanine. Cryst Growth Des. 2006;6:2441–5.
Madhurambal G, Mariappan M, Mojumdar SC. Thermal, UV and FTIR spectral studies of urea-thiourea zinc chloride single crystal. J Therm Anal Calorim. 2010;100:763–8.
Madhurambal G, Ramasamy P, Anbusrinivasan P, Vasudevan G, Kavitha S, Mojumdar SC. Growth and characterization studies of 2-bromo-40-chloro-acetophenone (BCAP) crystals. J Therm Anal Calorim. 2008;94:59–62.
Parthiban S, Murali S, Madhurambal G, Meenakshisundaram SP, Mojumdar SC. Effect of zinc(II)-doping on thermal and optical properties of potassium hydrogen phthalate (KHP) crystals. J Therm Anal Calorim. 2010;100:751–6.
Ramasamy G, Parthiban S, Meenakshisundaram SP, Mojumdar SC. Influence of alkali metal sodium doping on the properties of potassium hydrogen phthalate (KHP) crystals. J Therm Anal Calorim. 2010;100:861–5.
Muthu K, Bhagavannarayana G, Chandrasekaran C, Parthiban S, Meenakshisundaram SP, Mojumdar SC. Os(VIII) doping effects on the properties and crystalline perfection of potassium hydrogen phthalate (KHP) crystals. J Therm Anal Calorim. 2010;100:793–9.
Meenakshisundarm SP, Parthiban S, Madhurambal G, Mojumdar SC. Effect of chelating agent (1,10-phenanthroline) on potassium hydrogen phthalate crystals. J Therm Anal Calorim. 2008;94:21–5.
Mojumdar SC, Sain M, Prasad RC, Sun L, Venart JES. Selected thermoanalytical methods and their applications from medicine to construction. J Therm Anal Calorim. 2007;60:653–62.
Varshney G, Agrawal A, Mojumdar SC. Pyridine based cerium(IV) phosphate hybrid fibrous ion exchanger: Synthesis, characterization and thermal behaviour. J Therm Anal Calorim. 2007;90:731–4.
Madhurambal G, Ramasamy P, Anbusrinivasan P, Mojumdar SC. Thermal properties, induction period, interfacial energy and nucleation parameters of solution grown benzophenone. J Therm Anal Calorim. 2007;90:673–9.
Mojumdar SC. Thermoanalytical and IR-spectral investigation of Mg(II) complexes with heterocyclic ligands. J Therm Anal Calorim. 2001;64:629–36.
Yokotanic A, Sasaki T, Fujioka K, Nakai S, Yamanaka C. Growth and characterization of deuterated l-arginine phosphate monohydrate. A new nonlinear crystal for efficient harmonic generation of fusion experiment lasers. J Cryst Growth. 1990;99:815–9.
Naganathan PS, Venkatesan K. Crystal and molecular structure of glycyl-l-alanine hydrochloride. Acta Crystallogr B. 1972;55:131–5.
Ratajezak H, Baryck J, Pietraszko A, Debrus S, May M, Venturini J. Preparation and structural study of a novel nonlinear molecular material: L-histidinium dihydrogenarsenate orthoarsenic acid crystal. J Mol Struct. 2000;526:269–78.
Silverstein RM, Webster FX. Spectrometric identification of organic compounds. 6th ed. Canada: Wiley; 1998.
Colthup NB. Spectra-structure correlations in the infra-red region. J Opt Soc Am. 1950;40:397–400.
Joseph Arul Pragasam A, Madhavan J, Gulam Mohamed M, Selvakumar S, Ambujam K, Sagayaraj P. Growth and characterization of amino acid (glycine and valine) substituted l-arginine diphosphate single crystals. Opt Mater. 2006;29:173–9.
Kurtz SK, Perry TT. A powder technique for the evaluation of nonlinear optical materials. J Appl Phys. 1968;39:3798–813.
Meenakshisundaram SP, Parthiban S, Kalavathy R, Madhurambal G, Bhagavannarayana G, Mojumdar SC. Thermal and optical properties of ZTS single crystals in the presence of 1,10-phenanthroline (Phen). J Therm Anal Calorim. 2010;100:831–7.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Malliga, P., Alosious Gonsago, C., Sagayaraj, P. et al. Crystal growth, spectral, optical, and thermal characterization of glycyl-l-alanine hydrochloride (GLAH) single crystal. J Therm Anal Calorim 110, 873–878 (2012). https://doi.org/10.1007/s10973-011-1955-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-011-1955-1