Abstract
Resorcinol–formaldehyde aerogels (five samples, three of them with addition of nitrogen containing precursors—3-hydroxypyridine, 3-aminophenol and melamine) have been prepared by sol–gel polycondensation, subcritical drying and pyrolysis. The pyrolysis of prepared organic aerogels has been studied by non-isothermal TG at constant heating rate. The process of pyrolysis has been found to consist of three steps with the total mass loss 40.2–61.7% (room temperature—1,000 °C). The resulted carbon aerogels have been tested as sorbents of Ni(II), Pb(II) and Cu(II) ions from aqueous solutions. Various relations have been found among the results obtained from the pyrolysis experiments and properties affecting adsorption. Besides the expected correlation between the mass loss gained from TG (isothermal step at 500 °C was applied) and from heating in the laboratory oven, the relationship between the mass loss during pyrolysis and sorption capacities for all three metal ions has been found. Other relations among pyrolysis behaviour, surface area and content of nitrogen have been also examined. Batch adsorption experiments show (with an exception of one sample) that N-doped samples have higher adsorption capacity for metal ions. In addition, changing of nitrogen functionalities during the pyrolysis has been considered and pyridinic-N (N-6) functionality has been contemplated as a suitable structure for the adsorption process.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Organic aerogels are relatively new materials which were prepared in 1989 by Pekala and Kong [1] using polycondensation of resorcinol and formaldehyde. Aerogels are nanostructured materials, which contain up to 99 vol% of air [2]. Carbon aerogels are manufactured by the sol–gel technique by polymerisation and polycondensation [2], which is followed by drying and pyrolysis of organic matter to nanostructured carbonaceous material.
Carbon aerogels have wide range of applications such as electrodes in electrochemical capacitors and supercapacitors [3–5], nanocomposites (e.g. for photovoltaic materials) [6] or catalysts [7]. They may be also used in the field of hydrogen storage [8] and as adsorption material for removal of contaminants (e.g. heavy metals) from aqueous environment [9–11].
N-doped carbon aerogels can be prepared by adding the compounds which contain nitrogen (e.g. melamine [12–14], 3-hydroxyaniline [14], 3-hydroxypyridine [14], urea [15] and ammonia [8]) to the initial polycondensation mixture. The main reason for the adding nitrogen atoms to the structure of aerogel is to induce surface alkalinity, therefore, to enhance the adsorption properties [15]. The nitrogen containing precursor should be incorporated into aerogels in thermally stable forms [12].
The aim of this study was to investigate possible relation among results obtained from measurements of pyrolysis of prepared N-doped and non-doped organic aerogels and sorption properties of consequent (pyrolysed) carbonaceous materials.
Experimental
Sample preparation
Five samples of aerogels based on condensation of resorcinol (p.a.) (R) and formaldehyde (p.a., 37% solution, stabilized with methanol) (F) were prepared. HCl p.a., resp. Na2CO3 p.a. was used as catalyst for aerogels marked RFA, resp. RFB. Three nitrogen containing precursors—3-hydroxypyridine p.a. (HP), 3-aminophenol p.a. (AP) and melamine p.a. (M) were used for preparation of acid or alkali catalysed N-doped aerogels—RFA–HP, RFA–AP and RFB–M. Table 1 summarizes ratios of reactants used for the synthesis.
Solution A and solution B were prepared separately at room temperature (except for RFB–M solution A which was heated to 90 °C). The solutions were then mixed with intensive agitation, poured into glass vials and heated up to 70 °C for 1 h.
Wet organic gels were dried at 70 °C for 24 h at ambient pressure. Dry organic aerogels were pyrolysed in the flow of nitrogen (200 cm3 min−1) to obtain carbon aerogels. The heating programme consisted of an isothermal step at 100 °C (30 min), heating to 500 °C (10 K min−1) and keeping at the final temperature for 1 h.
Specific surface area of the prepared carbon aerogels was determined (at least twice) by N2 adsorption at −196 °C (BET).
Adsorption experiments
Batch sorption experiments were used to study adsorption capacities of the prepared carbon aerogels for three metal ions: Ni(II), Pb(II) and Cu(II). The stock solutions (5 mmol dm−3) were prepared by dissolving the appropriate amount of nitrates (p.a.) in demineralised water. Ratio of 0.5 g of aerogel to 50 cm3 of solution and contact time 24 h were used.
Concentrations of metal ions (before and after sorption) were determined by AAS (Varian FS 240). All experiments were repeated three times (average values are presented).
TG measurements
Thermogravimetry (Netzsch STA 449C) was used for studying the pyrolysis of the prepared organic aerogels in inert atmosphere (nitrogen, 100 cm3 min−1). Two temperature programmes were used. Heating from room temperature to 1,000 °C with heating rate 10 K min−1 enabled studying the whole pyrolytic process. The same heating extended with isothermal step (1 h at 500 °C) was applied in order to compare thermogravimetric results with analogous experiments in the laboratory oven.
The experiments were performed in crucibles from α-Al2O3, initial sample mass 19–21 mg (crushed to fine powders) was used in all measurements.
Results and discussion
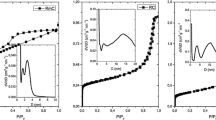
Comparison of aerogels appearance after polycondensation and after pyrolysis (Table 2) with the values of corresponding surface area (Fig. 1) shows that aerogels, which were evaluated as “compact” (RFA-AP and RFB), have the lowest values of surface area. Surface properties of other samples are in agreement with commonly published results for these materials [12–15].
Adsorption of Ni(II), Pb(II) and Cu(II)
The results of adsorption experiments show (Fig. 1) that sorption capacities for N-doped samples RFB–M and RFA–HP are higher than for non-doped samples RFB and RFA. The basic mechanism (connected with carbonaceous sorbents) is expected to be ion exchange [11], though there might be some additional mechanism for N-doped aerogels, which can enlarge sorption capacity (e.g. complexation).
Moreover, aerogel prepared by the adding 3-hydroxypyridine (RFA–HP) exhibits higher adsorption capacity than aerogel prepared with melamine (RFB–M). This can be explained by the fact that pyridinic N is thermally more stable than nitrogen atoms bonded in melamine, thus, more nitrogen atoms from HP can remain in the structure during the pyrolysis as possible adsorption sites [14]. RFA–AP showed different results, but its lower sorption capacity should be ascribed to low value of surface area and/or to the fact that the amino groups contained in AP are decomposed to gaseous products during the pyrolysis [14]. Finally, the presumption that presence of nitrogen atoms in the structure of carbon aerogel enhances the surface basicity and improves adsorption ability for metal ions can be confirmed.
According to the values of specific surface area (Fig. 1) samples can be divided into two groups. The first group of carbon aerogels (RFB and RFA–AP) is characterized by low surface area (below 100 m2 g−1) and low adsorption capacity. The second group (RFA, RFB-M and RFA-HP) exhibits much higher surface area and also higher adsorption capacity. However, there is no clear dependence (see Fig. 1) of adsorption capacity on value of surface area. The specific surface area is not crucial characteristic in adsorption of metals on prepared carbon aerogels. On the contrary, considerably low values of surface area can obviously limit diffusion of ions to adsorption sites and lead to lower sorption capacity even if the ion exchange or complexation mechanism is supposed.
Pyrolysis of organic aerogels (non-isothermal)
TG study of pyrolysis of organic materials is commonly used technique for characterization of these materials [2, 16–18]. Thermogravimetric study of pyrolysis of organic aerogels (example in Fig. 2) shows that three reaction steps can be distinguished. It is in compliance with literature [2, 16].
Step I (about 50–150 °C) is related to evaporation of water [2, 16]. Step II (200–450 °C) relates to the beginning of the pyrolysis when volatile components are being released [2, 16, 17]. This can be described as cracking of the C–O bonds (annealing reaction, outgassing of OH groups) [2]. The third step above 450 °C is connected with the pyrolysis itself leading to amorphous carbon [2, 16, 17].
The total mass loss during pyrolysis up to 1,000 °C varied in a relatively wide range from 40.2 to 61.7% (Table 3) but values published for analogous materials [2, 14] are in agreement with our results.
The relatively wide range of mass loss was found for every step of pyrolysis: step I (2.0–6.3%), step II (9.7–31.5%), even for step III (19.9–32.1%). These obvious variances can be associated with the presence of different N-containing precursors. It can be noticed that the difference in overlapping the steps II and III exists. These steps are well separated for RFA–HP and RFA–AP, while in the case of RFB–M it is difficult to accurately differentiate between volatile substances loss (step II) and the pyrolytic step III.
When we compare onset temperatures of pyrolysis (step II) we can conclude that the pyrolysis of acid catalysed organic aerogels begins at lower temperatures than alkali catalysed aerogels: 205, 291 °C resp. 292 °C for RFA–HP, RFA–AP resp. RFA, and 321 and 343 °C for RFB and RFB-M, respectively.
Finally, the pyrolysis of the sample RFA–HP is very different in comparison with the others. RFA–HP had the lowest humidity (step I), the highest mass loss of volatile components (step II), the highest total mass loss from both TG measurements and also from the laboratory oven (see next chapter). Nevertheless, this carbon aerogel has the highest adsorption capacity for metal ions.
Pyrolysis with isothermal step
Pyrolysis with the isothermal step at 500 °C (1 h) was performed in the laboratory oven and simultaneously measured with thermogravimetric apparatus. The mass loss at the end of the isothermal step for all samples from both laboratory oven and TG are presented in Table 4. The mass loss for pyrolysis in the laboratory oven was about 7% higher than for TG with the exception of RFA–HP, where values were nearly the same. Both values (oven and TG) correlate relatively well (R 2 = 0.96).
Evolution of nitrogen functionalities during pyrolysis
Several considerations about changes of nitrogen functionalities during pyrolysis can be based on literature data [19].
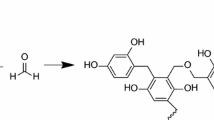
Nitrogen atoms in HP and M are bonded in 6-membered rings (N-6) and nitrogen atoms in AP and M in amino groups. We can suppose that during the preparation of the carbon aerogels the nitrogen atoms from precursors are incorporated in the final structure in 6-membered rings located at the edges of the graphene layers as pyridinic-N (N-6) or in the layer interior as quaternary-N (N-Q) [19]. Pels et al. [19] mentioned that after pyrolysis and exposure to the ambient atmosphere, N-oxides of pyridinic-N (N-X) can be formed and that during pyrolysis differences in nitrogen functionalities distribution of precursors had diminished. Apart from these forms also N-5 (nitrogen atom bonded in 5-membered rings) were found [8, 13, 14]. The amino groups do not appear in the carbonized aerogels due to their thermal decomposition [14]. Figure 3 shows possible structure of N-doped aerogel after pyrolysis.
It can be assumed (considering the studied samples), that amino groups originated from AP and M are decomposed during pyrolysis and that the nitrogen atoms in 6-membered rings of HP and M are transformed to pyridinic-N (N-6), quaternary-N (N-Q) or N-oxides of pyridinic-N (N-X). There is no reason for N-5 in the pyrolysed N-doped carbon aerogel in our samples.
The importance of the way how nitrogen atoms are bonded in the structure of aerogel for sorption properties is clear. N-Q and N-X forms are not able to coordinate with metal ions and affect the adsorption capacity, however, N-6 (and N-5, but not for our samples) functionalities on the periphery of graphene layers with a lone electron pair are active locations for metals adsorption. Long et al. [13] noticed that over two-thirds of the nitrogen atoms coming from precursors are on periphery and less than one-third is located inside of graphene layers for melamine doped aerogels.
Large loss of nitrogen content during pyrolysis was reported earlier [12, 13, 15]. Zhang et al. [12] also noticed that nitrogen atoms were released more easily than carbon atoms during pyrolysis. Gorgulho et al. [15] found in aerogels after pyrolysis only 20% of nitrogen added.
Relation between pyrolysis behaviour and sorption properties
As the mass loss increases during pyrolysis, adsorption capacities also increase for Ni(II), Pb(II) and Cu(II) ions (example in Fig. 4). This relationship can be noticed when adsorption capacities (a) and different mass losses are compared (a vs. mass loss from oven up to 500 °C with isothermal step, mass loss from TG up to 500 °C with isothermal step, mass loss from TG up to 1,000 °C with or without isothermal step).
These results are quite surprising, because the opposite relationships (increasing adsorption capacity with decreasing mass loss) have been expected. The correlation found can be probably explained by releasing the atoms, molecules, groups etc. which are “not connected” with sorption during pyrolysis. In other words, adsorption sites are “concentrated” with increasing amount of released gaseous products. Mass losses (and also adsorption capacities) were higher for two N-doped aerogels (RFA-HP and RFB-M), thus, it can be expected that nitrogen atoms are in these two cases incorporated in the structure to positions that are available for adsorption and in thermally stable form.
There is another relationship, which has been found between the surface area and theoretical content of nitrogen in the resulted carbon aerogels (surface area increases with theoretical content of nitrogen). This correlation could be random, because it has been derived only for the three samples and we have not determined the real content of nitrogen in the samples after pyrolysis.
Conclusions
Thermoanalytical study of pyrolysis of N-doped and non-doped organic aerogels has shown that some relation among parameters of pyrolysis and sorption properties of resulting carbon aerogels exists. The higher mass loss during pyrolysis leads to the higher sorption capacity for metal ions. Increasing mass loss during pyrolysis is probably accompanied with releasing of functionalities, which are not involved in adsorption process and consequently increasing “concentration” of adsorption sites on surface.
Various considerations about changing of nitrogen functionalities during preparation and pyrolysis have been made in connection with sorption abilities. It can be supposed that amino groups from N-precursors are decomposed during pyrolysis; however, nitrogen atoms in 6-membered rings are among others transformed to pyridinic-N (N-6) which is suitable for adsorption. Nevertheless, detailed study of the changes in nitrogen functionalities during preparation process would be beneficial to reveal which functionalities stay in the structure, and whether they are responsible for enhancing the sorption ability.
References
Pekala RW, Kong FM. A synthetic route to organic aerogels—mechanism, structure, and properties. J Phys Colloq. 1989;50:33–40.
Reuß M, Ratke L. Subcritically dried RF-aerogels catalysed by hydrochloric acid. J Sol-Gel Sci Technol. 2008;47:74–80.
Liu X-M, Zhang R, Zhan L, Long D-H, Qiao W-M, Yang J-H, Ling L-C. Impedance of carbon aerogel/activated carbon composites as electrodes of electrochemical capacitors in aprotic electrolyte. New Carbon Mater. 2007;22:153–8.
Lee YJ, Jung JC, Park S, Seo JG, Baeck S-H, Yoon JR, Yi J, Song IK. Effect of preparation method on electrochemical property of Mn-doped carbon aerogel for supercapacitor. Curr Appl Phys. 2011;11:1–5.
Jung H-H, Hwang S-W, Hyun S-H, Lee K-H, Kim G-T. Capacitive deionization characteristics of nanostructured carbon aerogel electrodes synthesized via ambient drying. Desalination. 2007;216:377–85.
Kim P-H, Kwon J-D, Kim JS. The impregnated synthesis of polypyrrole into carbon aerogel and its applications to photovoltaic materials. Synth Met. 2004;142:153–60.
Piao L, Chen J, Li Y. Carbon nanotubes via methane decomposition on an alumina supported cobalt aerogel catalyst. China Particuol. 2003;1:266–70.
Kang KY, Lee BI, Lee JS. Hydrogen adsorption on nitrogen-doped carbon xerogels. Carbon. 2009;47:1171–80.
Goel J, Kadirvelu K, Rajagopal C, Garg VK. Investigation of adsorption of lead, mercury and nickel from aqueous solutions onto carbon aerogel. J Chem Technol Biotechnol. 2005;80:469–76.
Kadirvelu K, Goel J, Rajagopal C. Sorption of lead, mercury and cadmium ions in multi-component system using carbon aerogel as adsorbent. J Hazard Mater. 2008;153:502–7.
Meena AK, Mishra GK, Rai PK, Rajagopal C, Nagar PN. Removal of heavy metal ions from aqueous solutions using carbon aerogel as an adsorbent. J Hazard Mater. 2005;B122:161–70.
Zhang R, Lu Y, Zhan L, Liang X, Wu G, Ling L. Monolithic carbon aerogels from sol–gel polymerization of phenolic resoles and methylolated melamine. Carbon. 2002;411:1645–87.
Long D, Zhang J, Yang J, Hu Z, Cheng G, Liu X, Zhang R, Zhan L, Qiao W, Ling L. Chemical state of nitrogen in carbon aerogels issued from phenol–melamine–formaldehyde gels. Carbon. 2008;46:1253–69.
Pérez-Cadenas M, Moreno-Castilla C, Carrasco-Marín F, Pérez-Cadenas AF. Surface chemistry, porous texture, and morphology of N-doped carbon xerogels. Langmuir. 2009;25:466–70.
Gorgulho HF, Gonçalves F, Pereira MFR, Figueiredo JL. Synthesis and characterization of nitrogen-doped carbon xerogels. Carbon. 2009;47:2032–9.
Xu G, Fang B, Sun G. Kinetic study of decomposition of wheat distiller grains and steam gasification of the corresponding pyrolysis char. J Therm Anal Calorim. 2011. doi:10.1007/s1097301116952.
Li C, Suzuki K. Kinetic analyses of biomass tar pyrolysis using the distributed activation energy model by TG/DTA technique. J Therm Anal Calorim. 2009;98:261–6.
Sarwar A, Khan MN, Azhar KF. Kinetic studies of pyrolysis and combustion of Thar coal by thermogravimetry and chemometric data analysis. J Therm Anal Calorim. 2011. doi:10.1007/s1097301117250.
Pels JR, Kapteijn F, Moulijn JA, Zhu Q, Thomas KM. Evolution of nitrogen functionalities in carbonaceous materials during pyrolysis. Carbon. 1995;33:1641–53.
Acknowledgements
The article has been done in connection with the project Institute of Environmental Technologies, reg. no. CZ.1.05/2.1.00/03.0100 supported by Research and Development for Innovations Operational Programme financed by Structural Founds of Europe Union and from the means of state budget of the Czech Republic.
Author information
Authors and Affiliations
Corresponding author
Additional information
3rd Joint Czech-Hungarian-Polish-Slovak Thermoanalytical Conference, Stará Lesná, Slovakia 2011.
Rights and permissions
About this article
Cite this article
Veselá, P., Slovák, V. Pyrolysis of N-doped organic aerogels with relation to sorption properties. J Therm Anal Calorim 108, 475–480 (2012). https://doi.org/10.1007/s10973-011-1949-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-011-1949-z