Abstract
Crystalline structure, thermo-oxidative and thermal stability of symmetrical and asymmetrical piperidyl and morpholinyl derivatives of both N-substituted and non-N-substituted butyl diphenyl-diketo-pyrrolopyrrole (DPP) pigments were studied using differential scanning calorimetry (DSC) and thermogravimetry (TG). Except for the asymmetrical morpholine DPP derivative, all the samples showed melting peaks which were relatively close to their degradation temperatures (from 260 to 430 °C). Using DSC, monotropic polymorphism was revealed in the symmetrical piperidyl-N-butyl-derivative which confirmed earlier observation about tendency of symmetrical N-alkyl DPP derivates to form several crystalline structures. TG carried out under nitrogen atmosphere served for distinguishing of evaporation/sublimation and degradation temperatures. Temperatures of evaporation/sublimation were typically 10–30 °C lower in comparison with temperatures of thermal degradation. The highest thermal (450 °C) and thermo-oxidative stability (around 360 °C) showed the DPP derivatives containing morpholine moieties with no alkyl substitution on NH-group of DPP core. The presence of the latter was found to be the most destabilizing factor. Piperidyl group showed more stabilizing effect due to its polar character and its influence on π–π intermolecular interactions of neighbouring phenyl groups. The highest stabilizing effect of morpholine moiety on DPP structure was explained based on the presence of polar oxygen atom in that group. The preparations of 3,6-di-(4-morpholinophenyl)-2,5-dihydro-pyrrolo[3,4-c]pyrrole-1,4-dione and 3-(phenyl)-6-(4-morpholinophenyl)-2,5-dihydropyrrolo[3,4-c]pyrrole-1,4-dione are reported.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Currently, a strong effort to produce organic electroluminescent devices (OLED) usable as a new generation of lamps and full colour flat panel displays, can be recognized. The potential advantages of these devices are their high efficiency, low driving voltage, versatility in their application (e.g. flexibility and transparency), low mass and relatively low production costs [1]. In order to fulfil all the requested parameters, the interest is focused not only on their optical properties, but also on physical–chemical characteristics such as solubility, resistance against various influences such as oxygen or temperature fluctuation.
3,6-diphenyl-2,5-dihydro-pyrrolo[3,4-c]pyrrole-1,4-dione known as DPP and its whole family of derivates are nowadays of a great interest [1–10]. Similarly, as other organic pigments they consist of centrosymmetric molecule having zero dipole moment [7]. Despite being of a relatively low molecular weight, DPP is an insoluble, crystalline material, thermally stable up to approximately 400 °C [8]. Physical properties are related to the presence of strong intermolecular bonds, which stabilize the crystal structure [1].
The derivatization of DPP is essential since resulted materials have improved physical–chemical properties such as for example, solubility, keeping the same or even better application properties in comparison with the parental DPP. Recently, it has been demonstrated that the introduction of piperidino (donor) and cyano (acceptor) groups into the DPP molecule had a strong influence on its absorption maxima. While both types of substitution resulted in bathochromic and hyperchromic shifts with respect to the parental DPP, the influence of piperidino group appeared to have the strongest effect [7].
Another example is a symmetrical dipyridyl derivate (DPPP) which shows a high proton affinity and, therefore, represents a good candidate for H2 gas sensors [9]. However, only one of two possible crystal phases of DPPP was shown to be sensitive for H2 detection; the reason of inactivity of one form is the interaction between N atom of pyridyl rings and NH-group(s) of DPP skeleton [9].
Thermal analysis represents a powerful tool in analysis of inorganic and organic pigments. The most important techniques are thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC), applied either separately, simultaneously or in combination with other techniques. Recently, those techniques were used in order to study the stability of DPP N-alkyl derivatives and polymorphism occurring upon fast and moderate cooling of the material [8].
More recently, the thermogravimetrical analysis of DPP derivatives of red-emitting diketopyrrolopyrrole-alt-phenylenevinylene [11] polymers and poly(arylene ethynylene)s derived from 1,4-diketo-3,6-diphenylpyrrolo[3,4-c] pyrrole [12] were reported. The thermal stability of the products were assessed in nitrogen atmosphere as ranging from 260 to 400 °C [11, 12].
The main aim of this study is the evaluation of the thermal and thermo-oxidative stability of DPP derivates with para-substituted phenyl groups and partially substituted NH-groups. Furthermore, DSC is employed in order to reveal possible phase transitions in heating run upon different cooling regimes. This should imitate the possible fluctuation of environmental conditions, which can hamper processing and possible applications of DPP derivatives. All parameters determined using thermo-analytical techniques are discussed with respect to the molecular structure of studied samples.
Experimental
Thermal analysis
TG studies were performed using TA Instruments TGA Q5000 (New Castle, DE, USA) device in 100-μL open platinum pans. The samples, typically around 5 mg, were heated by thermal ramp of 10 °C min−1 from 40 to 650 °C in a dynamic atmosphere of either nitrogen (thermal stability) or air (thermo-oxidative stability) of 25 mL min−1.
Calorimetric analyses were carried out employing TA Instruments DSC Q200 calorimeter equipped with an external cooler RCS90 allowing experimental temperature range from −90 to 500 °C. Experiments were conducted in open TA Tzero™ open aluminium pans. Thermal history of all samples was set up to be the same using a heating ramp of 10 °C min−1 from 40 °C to the temperature of 5 °C lower than the degradation onset, which was previously determined using thermogravimetry. Next, the moderate cooling ramp (0.5 °C min−1) was applied to reach −90 °C followed by 1 min of an isothermal stage. Next segment was performed using a heating ramp of 10 °C min−1 from −90 °C to the temperature of 5 °C lower than degradation onset. The last segment included a 10 °C min−1 heating run followed after the rapid equilibration of the sample down to −90 °C (quenching) and 1 min of isothermal stage. All DSC experiments were made under dynamic atmosphere of nitrogen, flow rate 50 mL min−1. Before the analyses, the device was calibrated for temperature and enthalpy using deionized water (own production), indium and tin standards (Perkin Elmer, Waltham, MA, USA.).
Samples
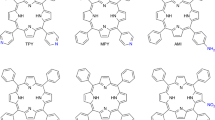
Five DPP derivates were investigated in this study. The molecular structures of tested DPP derivatives are reported in Fig. 1. The preparation of DPP1, DPP4 and DPP5 is reported in Ref. [9]. Preparation of DPP2 and DPP3 is given in following paragraphs.
Synthesis of 4-morpholine-1-yl-benzonitrile (starting nitrile)
Dry N,N-dimethylacetamide (p.a., 400 mL), p-fluorobenzonitrile (47.8 g; 0.4 mol) and morpholine (85 g; 0.98 mol) were charged into a 1-dm3 Erlenmeyer flask equipped with a stirrer and condenser. The reaction was carried out at 100–110 °C for 8 h. The reaction gases from the reaction were let out to fume-chamber. Subsequently, the reaction mixture was poured onto 1 kg ice. The crude product was collected by filtration and recrystallized from 80% ethanol. Yield was 38 g (50%) of 4-morfoline-1-yl-benzonitrile (m.p. 53–55 °C).
Synthesis of DPP2 (3,6-di-(4-morpholinophenyl)-2,5–dihydro-pyrrolo[3,4-c]pyrrole-1,4-dione)
Tert-amyl alcohol (390 mL) and sodium metal (24.4 g, 1 mol, in three portions) were charged into an 1.5-L Keller flask equipped with a stirrer, reflux condenser, thermometer and nitrogen inlet. The sodium metal was dissolved under reflux in the presence of catalytic amount of FeCl3 (which took approximately 2 h), and 4-morpholine-1-yl-benzonitrile (69 g, 0.37 mol) was added. After that, diisopropyl succinate (36.2 g, 0.18 mol) dissolved in tert-amyl alcohol (36.3 g) was added drop-wise within 3 h. Subsequently, this mixture was stirred under reflux for 1 h. The reaction mixture was cooled to 60 °C, and then 1000 mL of distilled water was added to protonate the salt. The protolysis was carried out at 80 °C for 2 h. The resulting hot suspension was filtered, and the filter cake was washed with hot water to neutral pH. The filter cake was dried and suspended in 800 mL acetone. The suspension was boiled and refluxed for 1 h. The hot suspension was filtered, washed with acetone and hot water. Yield of the DPP2 was 19.5 g (23.9%). Theoretical values of elemental analysis: C (68.11), H (5.72), N (12.22). Determined values of elemental analysis: C (67.89), H (5.75), N (12.05). 1H chemical shifts: (500 MHz, DMSO) 10.99 (2H, s, NH); 8.40 (4H, m, ArH); 7.11 (4H, m ArH); 3.78 (8H, t, H morpholine); 3.39 (8H, t, H morpholine).
Synthesis of DPP3 (3-(Phenyl)-6-(4-morpholinophenyl)-2,5–dihydropyrrolo[3,4-c]pyrrole-1,4-dione)
Tert-amyl alcohol (80 mL) and sodium metal (2.8 g, 0.12 mol) were charged into a 250-cm3 flask equipped with a stirrer, reflux condenser, thermometer and a nitrogen inlet. The sodium metal was dissolved under the reflux in the presence of catalytic amount of FeCl3 (which approximately took 2 h), whereupon 4-morpholine-1-yl-benzonitrile (7.5 g, 0.04 mol) was added. After that, pyrrolinone ester (9.2 g, 0.04 mol, in small portions) was continuously added within 0.5 h. Finally, this mixture was stirred and refluxed for 2 h. The hot suspension of sodium salt of compound DPP3 was collected by suction. The filter cake was charged into 200 mL propan-2-ol/water mixture (3/2 by vol), and the suspension was heated to boiling and refluxed for 2 h. The product was collected by filtration, and the filter cake was charged into 100 mL methanol and refluxed for a short time. The hot suspension was filtered, the filter cake was washed with methanol, and finally with hot water. Yield: 5.3 g (36%) compound DPP3. Theoretical values of elemental analysis: C (70.76), H (5.13), N (11.25). Determined values of elemental analysis: C (70.06), H (5.10), N (10.98). 1H chemical shifts: 500 MHz, DMSO) 11.19 (1H, s, NH); 11.16 (1H, s, NH); 8.46 (4H, m, ArH); 7.57 (3H, m ArH); 7.14 (2H, m ArH); 3.78 (8H, t, H morpholine); 3.39 (8H, t, H morpholine).
Results and discussion
The derivative TG results, i.e. DTG of samples measured under nitrogen and air are reported in Figs. 2 and 3, respectively. As it can be seen, the DTG curves are reported in the reverse direction then calculated in order to simplify the data reading. Table 1 summarizes the data obtained from TG, DTG and DSC analysis of DPP derivates. DTG–air and DTG–nitrogen–evaporation onset results show the onsets determined from DTG records by the approach indicated in the small frame in Fig. 2. While DTG–air has the meaning of thermo-oxidative stability, the DTG–nitrogen–evaporation onset indicates the temperature of intensive mass loss onset, i.e. it indicates either evaporation or sublimation. The character of the process, if either evaporation or sublimation takes place, is discussed in following paragraphs with respect to the obtained DSC results. The DTG–nitrogen–degradation onset stands for the thermal stability of a sample and it was determined from the second derivative as demonstrated in Fig. 4. This approach was developed and tested in Ref. [8]. Unlike for nitrogen experiments, the amount of char after air TG experiment is not reported since there was no rest after this kind of analysis. Regarding to the DSC results, Table 1 reports the enthalpies of melting and onset temperatures of respective events. For sample DPP2, the enthalpy of melting is not reported since the melting was immediately followed by the sample’s evaporation and, therefore, peak area of melting was overlapped by the peak of evaporation. For the sample DPP3, no melting peak before the DTG onset was recorded, therefore, the nitrogen DTG onset indicates the temperature of sublimation.
By applying TG [8], it was showed the onset temperature of sublimation of parental non-substituted DPP under nitrogen (sublimation) as high as 383 °C (for a heating rate 10 °C min−1). That process was followed by the thermal degradation at 408 °C and the thermo-oxidative stability was determined around 356 °C (under the same conditions as used in this study). Despite the fact that two crystallographic forms in parental DPP sample may occur, the DSC tests disclosed no significant phase transition such as melting or glass transition in the temperature interval from −90 to 383 °C. In contrast, the alkyl derivates (substitution of H in NH-group in parental DPP molecule) were less stable (a difference more than 100 °C) and their physical structure showed a strong dependence on the thermal pre-treatment (thermal history). That was explained as a consequence of a molecular symmetry disturbance caused by the presence of alkyl chains in the structure of DPP derivatives. As stated previously, pure DPP is stabilized by means of 3D intermolecular hydrogen bonds based on –NH···O=C interaction, by π–π intermolecular interactions between adjacent phenolic groups, by electrostatic interactions of charged groups and partly also by van der Waals forces [10]. Substitution of one or both H atoms in the NH-groups by alkyl chains in DPP molecule caused the destabilization of crystalline structure of derivatives under study. In fact, significant differences were found in physical structures of derivates depending on the symmetry and/or asymmetry of derivatization. Symmetrical derivatives exhibited a strong dependency on the thermal history, i.e. differences were recorded in the DSC heating run of the sample which was previously cooled either quickly or slowly. Such approach revealed the polymorphism of symmetrical derivates with alkyl chains –C4H9 and –C7H15 but not for –CH3. On the contrary, the asymmetrical derivates showed practically no response in physical structure when exposed to a different thermal history. It is noteworthy that glass transition was observed only in some samples regardless to the symmetry of the molecule.
Figure 2 reports the DTG of investigated samples in nitrogen. As already mentioned, the tests in nitrogen were carried out to obtain the temperatures of evaporation or sublimation; however, due to the intermolecular interactions stabilizing the DPP physical structure, processes of evaporation or sublimation are relatively slow and, therefore, the programmed continuous increase in the oven’s temperature causes later the degradation of the rest of the sample which was not removed yet and remained on the pan. In practise, when the sample is evaporated to be deposited on a surface, this problem can be solved by pressure reduction and keeping constant temperature during the deposition [10]. However, it can be seen that except for sample DPP5, all samples gave only one intensive DTG peak which indicates the one-step process. For this reason, the second derivative of TG signal was calculated in order to reveal some possible overlapping processes. Indeed, two separated processes were disclosed and attributed to the evaporation followed by the degradation, similarly as proved and published recently [8]. A typical determination is reported in Fig. 4.
The highest onset in nitrogen TG experiments showed samples DPP1 and DPP2 followed by samples DPP3 and DPP5. The lowest onset was observed for sample DPP4. In contrast, experiments in oxidative atmosphere of air brought about a different order. The highest stability was observed for sample DPP3, further for DPP2. Under oxidative conditions, sample DPP1 showed a lower stability than DPP2 (difference 25 °C) and it was only slightly higher than the stability of sample DPP5. Again, the lowest stability was observed for sample DPP4.
Differences in stabilities under different conditions were accompanied also by differences in physical structures as revealed by DSC measurement. It can be identified in Table 1 that melting temperatures of samples DPP1, DPP2 and DPP5 are higher than DTG–air onset temperatures which suggest that samples underwent thermo-oxidative degradation before their melting. On the contrary, all the samples (except DPP3) were pre-melted before the onset determined by DTG under inert conditions.
In general, it can be stated that all DPP derivates investigated in this study showed higher stability than N-alkyl derivatives tested in Ref. [8]. In contrast, as follows from the above-statements, the parental DPP showed higher thermal stability than DPP5 and DPP4; the thermo-oxidative stability was higher than DPP5, DPP4, DPP1 and similar to DPP2.
Unlike the degradation in nitrogen, the degradation in oxidative atmosphere (Fig. 3) proceeded in several steps. The lowest stability showed samples with aliphatic chains in the structure. In fact, the mass loss extracted from TG curve in the temperature range corresponding to the first degradation step in sample DPP4 was about 20% which is equal to the molar fraction of alkyl part in that molecule. Analogous result can be obtained also for sample U5, i.e. detected mass loss was 11%. Therefore, it seems that the less stable part of the derivative molecule is the aliphatic chain attached to the N-molecular skeleton of DPP. The piperidine part of a molecule has less de-stabilizing effect, probably due to its polar character and its influence on π–π intermolecular interactions of phenyl groups. Employing the same approach as for aliphatic derivates for sample DPP1, the piperidyl group is decomposed in several steps. Figure 3 shows two identical peaks at 340 and 349 °C corresponding to 9 and 20% mass loss, respectively. At 37% of degradation (i.e. when both piperidyl groups are degraded) at 450 °C which corresponds to the minimum between two main degradation stages. Piperidyl group is more resistant against thermo-oxidative attack than simple aliphatic chain. This can be easily identified also when the most stable sample DPP1 (with no alkyl chain) is compared with DPP4 (symmetrical N-alkyl derivatization), which is less stable and with DPP5 (asymmetrical N-alkyl derivatization), which is only slightly less stable in air, however, significantly less stable in nitrogen. Such order also supports the observation which was done in the study with N-alkyl derivates that asymmetrical derivates of DPP have higher stability than the symmetrical ones.
Comparison of samples DPP1 and DPP2, i.e. samples with different para-substituents on phenyl groups of DPP showed only slight difference in thermo-oxidative stability while thermal stability was identical. The piperidyl groups showed lower stabilizing effect against oxidation than morpholine group which can be explained as a stabilizing effect of polar O atom in morpholine group. However, as indicated by DSC (Table 1) there is a similar influence of both substituents on melting temperature in both derivatives. Since parental DPP did not show any melting before the evaporation [8] it can be assumed that both substituents, donors of electrons, have an influence on the weakening or opening of the structure allowing the oxygen molecules to penetrate inside the structure and decompose the material. The degradation of morpholine groups in sample DPP2 above-mentioned approach, based on mass loss calculation, failed, which implies that the breaking down of that group proceeds in a more complicated way than aliphatic chains or piperidyl groups. Interestingly, such approach was successful in sample DPP3 where the mass loss of morpholine group corresponded to the minimum in DTG after the first degradation step.
Comparison of DSC records of DPP1 and DPP4 can shed an additional light on the influence of aliphatic chains on the stability of DPP derivatives structure. As it can be seen, the alkyl chains destabilize significantly the structure and cause the lower stability of crystals in sample DPP4. Significantly higher melting enthalpy of sample DPP1 indicates the formation of completely different crystalline structure than in the case of N-alkyl derivates (their enthalpy of melting) was reported as significantly lower than those reported in Ref. [8].
Differences between samples DPP3 (asymmetrical substitution of DPP’s phenyls) and DPP2 (symmetrical substitution) brought the insight into the influence of dipole moment on their thermal and thermo-oxidative stability. In contrast to DPP2, due to its asymmetry, the molecule DPP3 has non-zero dipole moment [13] stabilizing the structure against oxygen agitation. It is noteworthy that DPP3 is the only sample which had no melting before the DTG onset. Therefore, the present dipole moment destabilizes the structure and the sublimation (thermal stability) of sample DPP3 occurs at lower temperatures than for DPP 2.
As reported in Fig. 5, sample DPP4 showed a strong dependency on the thermal pre-treatment. When heated up without any pre-treatment, indicated as ‘C’ record in Fig. 4 (i.e. measured as received), there can be seen three melting events (in Table 1 only the last peak, the most intensive one is reported). Small peaks occurred at 153 and 166 °C with respective enthalpies of 0.7 and 11.7 J g−1. On the other hand, when cooled quickly, indicated as ‘A’ record, a glass transition with midpoint at 58 °C accompanied by a slight enthalpic recovery appeared followed by two exotherms attributable to crystallization. Peaks occurred at 110 and 164 °C with enthalpies 39.8 and 5.1 J g−1, respectively. Melting of present crystalline structure was observed at 263 °C. Finally, when cooled slowly, only the melting at 254 °C was observed with no pre-crystallization event (‘B’ record).
Such behaviour suggests the possible polymorphism of sample DPP4. As mentioned in previous paragraphs, similar, but not the same, temperature-dependent behaviour was observed for symmetrical N-alkyl DPP derivates, namely C4H9 and C7H15 derivatives [8]. That was attributed to the melting of two different crystalline forms implying the monotropic polymorphism. The possible existence of several crystalline structures was observed only in the first ‘C’ record. The run ‘A’ which shows also the glass transition can be explained as a consequence of quick cooling; the movement of molecules otherwise forming crystals is decelerated and instead, they form amorphous structures. Heating up of the sample causes molecular segments relaxation (glass transition) followed by two steps of crystal perfection. Since aliphatic groups are involved in the crystalline structure, two phases may imply the progressive formation of both aliphatic and piperidyl moieties containing crystalline domains.
Conclusions
Thermal analysis has already showed its potential to study physical–chemical properties of pigments and dyes, e.g. refs. [8, 14, 15]. In this study, the physical–chemical properties of DPP derivates were investigated employing thermal analysis methods. The determined temperatures, especially the distinguishing between evaporation/sublimation and degradation temperatures is important for the designing of experimental conditions for deposition of such materials in the form of thin layers. The high thermal and thermo-oxidative stability of DPP materials which was determined using TG is a promising factor supporting their future application. Furthermore, the knowledge on the physical structure, as revealed by DSC showed the potential problem in manipulating with some DPP derivates since only an appropriate crystalline structure can be requested for the specific application.
References
Vala M, Weiter M, Vyňuchal J, Toman P, Luňák S Jr. Comparative studies of diphenyl-diketo-pyrrolopyrrole derivatives for electroluminescence applications. J Fluoresc. 2008;18:1181–5.
Mizuguchi J. Correlation between crystal and electronic structures in diketopyrrolopyrrole pigments as viewed from exciton coupling effects. J Phys Chem A. 2000;104:1817–21.
Hoki T, Takahashi H, Suzuki S, Mizuguchi J. Hydrogen gas sensor based upon proton acceptors integrated in copper-tetra-2, 3-pyridinoporphyradine. IEEE Sensors J. 2007;7:808–13.
Beyerlein T, Tieke B, Forero-Lenger S, Brütting W. Red electroluminescence from a 1, 4-diketopyrrolo[3, 4-c]pyrrole (DPP)-based conjugated polymer. Synthetic Metals. 2002;130:115–9.
Potrawa T, Langhals H. Fluorescent dyes with large Stokes shifts - soluble dihydropyrrolopyrrolediones. Chemische Berichte. 1987;120:1075–8.
Fukuda M, Kodama K, Yamamoto H, Mito K. Evaluation of new organic pigments as laser-active media for a solid-state dye laser. Dyes Pigments. 2004;63:115–25.
Luňák S, Vyňuchal J, Vala M, Havel L, Hrdina R. The synthesis, absorption and fluorescence of polar diketo-pyrrolo-pyrroles. Dyes Pigments. 2009;82:102–8.
David J, Weiter M, Vala M, Vyňuchal J, Kučerík J. Stability and structural aspects of diketopyrrolopyrrole pigment and its N-alkyl derivatives. Dyes Pigments. 2011;89:137–43.
Vala M, Vyňuchal J, Toman P, Weiter M, Luňák S Jr. Novel, soluble diphenyl-diketo-pyrrolopyrroles: Experimental and theoretical study. Dyes Pigments. 2010;84:176–82.
Weiter M, Salyk O, Bednář P, Vala M, Navrátil J, Zmeškal O, Vyňuchal J, Luňák S Jr. Morphology and properties of thin films of diketopyrrolopyrrole derivatives. Mat Sci Eng B. 2009;165:148–52.
Qiao Z, Xu Y, Lin S, Peng J, Cao D. Synthesis ands characterization of red-emitting diketopyrrolopyrrole-alt-phenylenevinylene polymers. Synthetic Metals. 2010;160:1544–50.
Palai AK, Mishra SP, Kumar A, Srivastava R, Kamalasana MP, Patri M. Synthesis and characterization of alternative donor-acceptor arranged poly(arylene enthylene)s derived from 1, 4-diketo-3, 6-diphenylpyrrolo[3, 4-c]pyrrole (DPP). Eur Polym J. 2010;46:1940–51.
Mizuguchi J, Imoda T, Takahashi H, Yamakami H. Polymorph of 1, 4-diketo-3, 6-bis-(4′-dipyridyl)-pyrrolo-[3, 4-c]pyrrole and their hydrogen bond network: A material for H2 gas sensor. Dyes Pigments. 2006;68:47–52.
Rotaru A, Moanta A, Popa G, Rotaru P, Segal. E. Thermal decomposition kinetics of some aromatic azomonoethers. full access. Part IV. Non-isothermal kinetics of 2-allyl-4-((4-(4-methylbenzyloxy)phenyl)diazenyl)phenol in air flow. J Therm Anal Calorim. 2009;97:485–91.
Rotaru A, Moanta A, Rotaru P, Segal E. Thermal decomposition kinetics of some aromatic azomonoethers Part III. Non-isothermal study of 4-[(4-chlorobenzyl)oxy]-4′-chloroazobenzene in dynamic air atmosphere. J Therm Anal Calorim. 2009;95:161–6.
Acknowledgements
The financial support of the Ministry of Education of the Czech Republic - project MSM 0021630501, Academy of Sciences of the Czech Republic project KAN401770651 and Czech Science Foundation project GACR 203/08/1594 are acknowledged. This study was also supported by the project “Centre for Materials Research at FCH BUT” No. CZ.1.05/2.1.00/01.0012 from ERDF.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kučerík, J., David, J., Weiter, M. et al. Stability and physical structure tests of piperidyl and morpholinyl derivatives of diphenyl-diketo-pyrrolopyrroles (DPP). J Therm Anal Calorim 108, 467–473 (2012). https://doi.org/10.1007/s10973-011-1896-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-011-1896-8