Abstract
The infrared spectrum of polycaprolactone has been recorded as a function of temperature in the range where melting and crystallisation of the polymer can occur. Examination of the carbonyl band of the spectra reveals a clear morphological sensitivity; heating the semi-crystalline polymer through the melting region results in a decrease in the intensity of the crystalline component of the carbonyl band. Accordingly, there was a subsequent increase in intensity of the crystalline carbonyl band on cooling. To enable comparison of these findings with a more conventional method of thermal analysis, similar experiments were conducted using a differential scanning calorimeter. The heated ATR accessory adopted for use in the FTIR spectrometer imposed significant limitations in the range of possible heating and cooling rates, but when these rates were carefully matched between FTIR and DSC, close correlation between the melting point and onset of re-crystallisation was observed. The results confirm that FTIR can be used as an alternative, if more laborious, way of investigating melting and re-crystallisation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polycaprolactone (PCL) is a semi-crystalline aliphatic polyester. Both biocompatible and biodegradable PCL is used in a wide range of applications including tissue engineering scaffolding, bioabsorbable sutures and macromolecular drug release in the human body [1, 2]. PCL typically has a glass transition temperature of −60 °C and exhibits a melting peak in the region of 60 °C. The temperature at which the crystallisation process proceeds most rapidly is in the region of 30 °C [3]. Understanding the crystallisation process and its effect on crystallinity is important as this characteristic in a polymer can control material properties such as stiffness, strength and the biodegradation rate [4].
Characterisation of the degree of crystallinity is conventionally investigated using techniques such as differential scanning calorimetry (DSC), X-ray analysis and density measurements. The kinetics of the crystallisation process are readily measured by DSC. However, in recent years, the use of infrared spectroscopy as a method of characterisation of crystallinity has grown in prominence. In the case of PCL, one of the first and often cited studies concerned the study of a PCL/PVC blend system using FTIR spectroscopy [5]. In this work, the authors report on the morphological origin of the prominent carbonyl band in the PCL component. The PCL carbonyl band was shown to be split into two components, a crystalline band at 1724 cm−1 and an amorphous band at 1737 cm−1. The authors conclude by suggesting that FTIR provides a means of studying the crystallisation process via spectroscopic means and that rapid acquisition of data offers a way of exploring the early stages of crystallisation. However, it is also noted that measurements of crystallinity determined using FTIR should also be correlated with other techniques such as X-ray spectroscopy.
While other papers followed in the area of PCL blends [6, 7], the next study to focus solely on the exploration of crystallinity of PCL using FTIR was that reported by Yong et al. [8]. In this work, the carbonyl band was used to determine a measure of the extent of crystallinity. Good agreement with literature values from other techniques including X-ray and NMR were reported. However, related studies concentrating on the detection of the melting process and non-isothermal crystallisation in PCL using FTIR have received little or no attention in the literature. Therefore, the purpose of this paper is to extend the scope of earlier studies by adopting a time-resolved approach to the detection of melting and crystallisation processes in PCL and, in particular, to correlate the processes of melting and crystallisation as detected by FTIR with the conventional technique of differential scanning calorimetry. It will be shown that in the case of non-isothermal crystallisation, there is good agreement between melting and crystallisation processes measured using FTIR and DSC.
Experimental
Materials
Polycaprolactone (CAPA 6800) was supplied in pellet form by Solvay Interox Ltd (UK). The number and weight average molecular weights of CAPA6800 were 69000 and 120000, respectively (manufacturer’s data). Plaques of thickness 0.3 mm were produced by compression moulding at 150 °C using a heated press. The plaques were removed from the press and allowed to cool to room temperature. Circular samples with a diameter of 4 mm were cut from the plaque using a hole-punch. The sample mass was typically 6 mg.
Differential scanning calorimetry (DSC)
The melting and crystallisation behaviour of PCL was measured using a Perkin Elmer differential scanning calorimeter (DSC 7) interfaced to a personal computer. The thermal response of the instrument was calibrated from the enthalpy of fusion of a known mass of indium (99.999% pure). The temperature of the calorimeter was calibrated using the melting points of benzoic acid, stearic acid and octadecane. Corrections were made for thermal lag by extrapolation to zero heating rate. Plots of actual versus experimental melting points were linear and were used to calibrate the calorimeter temperature.
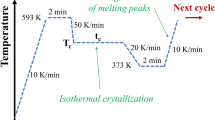
Samples of 6 mg were contained in aluminium pans, and an empty pan was used as a reference. Samples were initially heated from 20 to 100 °C at 20 °C min−1 and then cooled back to 20 °C at 20 °C min−1 to enable the creation of a known thermal history within the sample. Melting traces were recorded at a low heating rate of 0.5 °C min−1 to match the heating rates imposed by the infrared spectroscopy experiments. Non-isothermal crystallisation was achieved by cooling from the melt at 0.5 °C min−1. Again, very slow cooling rates were selected to match the rates imposed by the limitations of the temperature control of the ATR unit on the FTIR spectrometer.
Isothermal crystallisation was achieved by heating the samples to 5 °C above the observed melting point, holding for 2 min and then cooling to the selected crystallisation temperature of 46 °C for 120 min. To minimise crystallisation during the cooling phase, crystallisation temperatures close to the melting point were selected. The relative crystallinity was calculated according to the following expression,
in which (dH/dT) is the relative heat flow rate.
Infrared spectroscopy
Changes to the infrared spectrum of PCL during either heating or cooling were recorded using a Nicolet 860 FTIR. Measurements were made using a heated Golden Gate ATR supercritical fluids analyser supplied by Specac (p/n 10585). The temperature was controlled and monitored by a dedicated heater control unit. A thermocouple and digital temperature sensor were used to calibrate the temperature of the ATR cell. A plot of actual versus control temperature was constructed and used to calibrate the temperature of the cell. Infrared spectra were recorded at a resolution of 2 cm−1 and 200 scans. This combination was found to be the best compromise between high spectral quality and minimum acquisition time.
Samples of known thermal history were created using DSC (by heating the sample to above the melting point and cooling to room temperature at 20 °C min−1) and extracted from the DSC pans prior to placement on the ATR cell. To detect the melting process, the infrared spectrum of PCL was recorded as a function of temperature by heating the semi-crystalline samples from ambient temperature to 70 °C at 0.5 °C min−1. To detect the non-isothermal crystallisation process, the resulting amorphous samples were then cooled from the melt to room temperature at 0.5 °C min−1. This relatively slow cooling rate was imposed by the lack of forced cooling on the ATR cell. Isothermal crystallisation was detected by heating a semi-crystalline PCL sample to 70 °C and then cooling at 0.5 °C min−1 to 46 °C. The rationale for the selection of this temperature was to ensure the crystallisation process occurred within a reasonable timescale, but did not initiate before the isothermal crystallisation temperature was reached.
Results and discussion
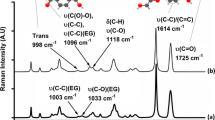
The carbonyl region of the FTIR spectrum of PCL is shown in Fig. 1. It is clear that the band is composed of two separate, but overlapping peaks. There is a relatively broad band centred at 1737 cm−1 and a sharper and more intense band at 1724 cm−1; it has been shown previously that the origin of the above peaks can be traced to the amorphous and crystalline phases of the polymer, respectively [9, 10]. The amorphous band is relatively broad due to the wider range of possible local environments of the carbonyl group in the amorphous phase. The situation in the crystalline phase is different due to the higher degree of ordering, which leads to a reduction in the number of local environments and hence a sharper peak is observed. A further consequence of the high degree of ordering in the crystalline phase is the presence of inter and intramolecular forces. These forces affect the dipole moment of the carbonyl groups bound into the crystalline lamellae and the associated band shifts to a lower wavenumber.
On heating from 30 to 70 °C, the intensity of the amorphous and crystalline peaks in the carbonyl band was found to vary. The most prominent change was found in the crystalline band at 1724 cm−1 where the intensity of the band decreased from an initial absorbance of 0.77 at 30 °C to 0.59 at 70 °C. Due to the overlapping nature of the carbonyl peaks, it was not possible to measure the absorbance of the crystalline band above 55 °C, as beyond this temperature the crystalline peak became fully obscured by the amorphous band, which increased in intensity as the temperature increased. Given that the carbonyl band is in effect diagnostic of the morphology of the sample under investigation, the changes in band intensity shown in Fig. 1 can be attributed to the melting process.
The variation in the intensity of the crystalline carbonyl band with temperature is shown in Fig. 2. The most striking feature of the figure is the close correlation of the sharp decrease in crystalline carbonyl absorbance in the region 50–56 °C with the melting point of the polymer as detected by DSC. Clearly, the heating rate adopted in the DSC experiment will affect the temperature at which melting is observed, but in the case of the comparison shown in Fig. 2, the heating rates were matched at 0.5 °C min−1 to minimise the effects associated with thermal lag. Various comparisons between the traces shown in Fig. 2 are possible. These include the extrapolated onset of melting, the peak melting temperature and the temperature corresponding to the last trace of crystallinity.
The extrapolated onset of melting from DSC was found to be 51.8 °C. By applying a similar geometrical construction to the variation of crystalline carbonyl peak intensity with temperature, the onset of melting as detected by FTIR was found to be 51.5 °C. The last trace of crystallinity detected by DSC and FTIR was 55.2 and 56.1 °C, respectively. The peak melting temperature measured by DSC was found to be 53.7 °C, but no corresponding measure from FTIR was possible due to the fact that the crystalline carbonyl peak became obscured by the amorphous band and therefore impossible to resolve with the apparatus required for the experiment. The difference between the above measures is limited to a maximum of 0.9 °C, which can easily be attributed to a combination of the error in the measurement of temperature and subtle differences in the thermal lag characteristics of both techniques. Therefore, providing that the heating rates are matched, it appears that FTIR offers an alternative (if rather more laborious) method to DSC for the detection and measurement of the melting process in PCL.
It is worth noting the decrease in absorbance from 30 to 50 °C, which occurs prior to the main melting process. This decrease in absorbance may suggest the melting of relatively thin lamellae, but a more reasonable interpretation can be found in the effect of thermal expansion of the sample on heating, which effectively reduces the number of molecules within the sampling area of the beam and results in a progressive decrease in absorbance.
The effect of cooling PCL from the melt on the carbonyl band is shown in Fig. 3. A strong similarity to Fig. 1 can be observed in that the band is clearly temperature dependent, but in the case of Fig. 3, on cooling there is a progressive growth in the crystalline component of the carbonyl band and a corresponding (but much less pronounced) decrease in the amorphous component of the band. This variation can be ascribed to the process of crystallisation on cooling. The variation of the intensity of the crystalline component of the band with temperature is shown in Fig. 4. For comparison, the corresponding DSC cooling curve is also shown.
Analysis of the curves shown in Fig. 4 reveals a strong correlation between the onset of crystallisation process as detected by DSC and FTIR. In the case of DSC, the temperature at which the crystallisation exotherm becomes apparent is 41.7 °C. In the case of FTIR, the onset of the crystallisation process can be defined as the point at which the first data point corresponding to the crystalline carbonyl band can be resolved; this can be shown to be 43 °C. If an uncertainty of ± 1 °C is assumed in the measurement of temperature in the heated ATR unit, then the techniques of DSC and FTIR can be said to correlate reasonably well in the detection of the onset of crystallisation. However, there is a clear dissimilarity in the breadth of the crystallisation process as detected by these techniques.
The difference in the breadth of the crystallisation process as detected by DSC and FTIR may be explained by a difference in the thermal characteristics of the DSC cell and the heated ATR unit. In the case of the heated ATR unit, the FTIR spectrometer samples only a fraction of the crystallising polymer (in the case of the unit adopted for this study, the sample area is limited to an area of 2 mm2 and the sampling depth is in the region of 2 microns). In the case of DSC, the sample thickness is far in excess of this at 0.3 mm, so the effects of thermal lag are likely to be far more pronounced, causing an increase in the breadth of the transition.
The effect of isothermal crystallisation on the carbonyl band of the PCL FTIR spectrum is illustrated in Fig. 5; the crystallisation temperature was 46 °C. The response is almost identical to that shown in Fig. 3 in that the crystalline component of the band increases with time. There is also a corresponding decrease in the intensity of the amorphous band with time. Furthermore, it is also apparent from Fig. 5 that there is evidence of a well-defined isosbestic point, indicating that the conversion of amorphous to crystalline material at this temperature does not involve an intermediate species. While Figs. 1 and 3 also show evidence of isosbestic points, they are less pronounced. Since these figures show FTIR data gathered under non-isothermal conditions, it is likely that the absorption coefficients of the crystalline and amorphous phases of semi-crystalline PCL exhibit some degree of temperature dependence and thus a loss of clarity in the definition of this point.
Figure 6 shows the variation of the normalised peak intensity of the crystalline carbonyl band with time (crystallisation temperature 46 °C). Also shown in Fig. 6 is the variation of relative heat flow with time at the same isothermal crystallisation temperature. It is clear that both FTIR and DSC detect the transformation of amorphous to crystalline material on a similar timescale. However, closer inspection of the half-lives reveals a difference in that after subtraction of the induction times for the crystallisation processes, the half -lives for FTIR and DSC were found to be 40 and 31 min, respectively. This variation in half-life may be due to inherent differences in the sensitivities of the techniques, one which warrants further investigation.
Conclusions
A clear morphological sensitivity of the carbonyl band in PCL has been observed. The variation of the crystalline component of the band with temperature has been compared with that of relative heat flow obtained from DSC and a good correlation has been found. Furthermore, the crystallisation process detected using FTIR spectroscopy was observed to be sharper than the corresponding exotherm observed using DSC. This observation was interpreted in terms of reduced thermal lag in the ATR cell due to a much smaller effective sample volume. Despite showing some technical advantages, this work has shown that FTIR spectroscopy is a cumbersome method to determine the crystallinity and crystallisation in PCL and that DSC is a far more practical approach to measurements of this kind.
References
Zhu G, Xu Q, Qin R, Yan H, Liang G. Effect of [gamma]-radiation on crystallization of polycaprolactone. Radiat Phys chem. 2005;74:42–50.
Hutmacher DW. Scaffolds in tissue engineering bone and cartilage. Biomaterials. 2000;21:2529–43.
Acierno S, Di Maio E, Iannace S, Grizzuti N. Structure development during crystallization of polycaprolactone. Rheol Acta. 2006;45:387–92.
Jenkins MJ, Harrison KL. The effect of crystalline morphology on the degradation of PCL in a solution of PBS and lipase. Polym Adv Technol. 2008;19:1901–6.
Coleman MM, Zarian J. Fourier-transform infrared studies of polymer blends. II. Poly(ε-caprolactone)-poly(vinyl chloride) system. J Polym Sci. 1979;17:837–50.
Wang J, Cheung MK, Mi Y. Miscibility and morphology in crystalline/amorphous blends of poly(caprolactone)/poly(4-vinylphenol) as studied by DSC, FTIR, and 13C solid state NMR. Polymer. 2002;43:1357–64.
Jiang H, Wu P, Yang Y. Variable Temperature FTIR Study of Poly(ethylene-co-vinyl alcohol)-graft-poly(ε-caprolactone). Biomacromolecules. 2003;4:1343–7.
Yong H, Yoshio I. Novel FTIR method for determining the crystallinity of poly(ε-caprolactone). Polym Int. 2000;49:623–6.
Xu J, Guo B-H, Yang R, et al. In situ FTIR study on melting and crystallization of polyhydroxyalkanoates. Polymer. 2002;43:6893–9.
Kansiz M, Domínguez-Vidal A, McNaughton D, Lendl B. Fourier-transform infrared (FTIR) spectroscopy for monitoring and determining the degree of crystallisation of polyhydroxyalkanoates (PHAs). Anal Bioanal Chem. 2007;388:1207–13.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Murphy, S.H., Leeke, G.A. & Jenkins, M.J. A Comparison of the use of FTIR spectroscopy with DSC in the characterisation of melting and crystallisation in polycaprolactone. J Therm Anal Calorim 107, 669–674 (2012). https://doi.org/10.1007/s10973-011-1771-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-011-1771-7