Abstract
The pyrolysis characteristics and kinetics of sewage sludge for different sizes (d < 0.25 mm, 0.25 mm < d < 0.83 mm, and d > 0.83 mm) and heating rates (5, 20, and 35 °C/min) were investigated in this article. The STA 409 was utilized for the sewage sludge thermogravimetric analysis. FTIR analysis was employed to study the functional groups and intermediates during the process of pyrolysis. Meanwhile, a new method was developed to calculate pyrolysis kinetic parameters (activated energy E, the frequency factor A, and reaction order n) with surface fitting tool in software MATLAB. The results show that all the TG curves are divided into three stages: evaporation temperature range (180–220 °C), main decomposition temperature range (220–650 °C), and final decomposition temperature range (650–780 °C). The sewage sludge of d < 0.25 mm obtains the largest total mass loss, especially at the heating rate of 5 °C/min. By FTIR analysis, the functional groups including NH, C–H, C=C, etc., are all found in the sewage sludge. There is a comparison between the FTIR spectra of sludge heated to 350 °C (temperature associated to maximum devolatilization rate in the second stage) and the FTIR spectra of sludge heated to 730 °C (temperature associated to maximum devolatilization rate in the third stage). In the second stage, the alcohols, ammonia, and carboxylic acid in the sludge have been mostly decomposed into gases, and only a little bit of compounds containing CH and OH of COOH exist. The pyrolysis kinetic parameters of second stage are as follows: the reaction orders are in the range of 1.6–1.8 and the activation energy is about 45 kJ/mol. The frequency factor increases with the increase of heating rate and sewage sludge size.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sewage sludge is produced in significant quantities during the industrial and domestic waste water treatment by biological processes and growing sharply in recent years. Consequently, many treatment processes were developed for sewage sludge, such as landfill, dumping to the sea, directly, or composted as fertilizer on agricultural land and thermal treatment (combustion or incineration). However, these conventional methods also bring about some other problems during the course. For example, landfill occupies large space and contaminates ground water. Dumping to the sea brings about sea pollution. Directly or composted as fertilizer on agricultural land produces secondary pollution as a result of toxic metals and organic compounds. And combustion or incineration leads to air pollution and ash treatment because of toxic metals.
The requirements of the governmental legislation for the disposal and usage of sewage sludge coupled with the increase of their generation and decreasing acceptance of traditional routes for their disposal have led to a search for new alternative uses for sewage sludge [1]. Pyrolysis is one of the alternatives for the treatment of sewage sludge. Pyrosis presents the advantages of concentrating on the heavy metals (except mercury and cadmium) and converting the stored energy into electrical power and valuable chemicals, and so on. The accurate qualitative and semi-quantitative gas phase analysis released compounds in the pyrolysis process [2–4]. Meanwhile, to know the intrinsic of sewage sludge pyrolysis, the important task is to study its kinetics.
Numerous literatures have reported kinetic data for fossil fuels, alternative fuels, and several solid wastes [5–11]. Conesa with two types of sewage sludge (before and after the anaerobic treatment in an aerobic-activated sludge waste water plant) deduced that the best model for both the sludges considers three different organic fractions [11]. He et al. proposed a four reaction model with two serial competitive reactions, producing volatile matter and char, respectively [12]. Stolarek and Ledakowicz analyzed the drying pyrolysis process considering six fractions of lumps, assuming first order reactions [13]. L.F. Calvo offered the kinetic data for sewage sludge heating in different atmospheres [14]. Lech Nowicki did a research on kinetics of gasification of char derived from the sewage sludge under different oxidizing atmospheres containing CO2, O2, or H2O [15]. Mohd Roslee Othman analyzed thermogravimetric characteristics and pyrolysis kinetics of the Giheung Respia sewage sludge [16]. However, no one offered the kinetic data of the sewage sludge for different sizes. In this study, three sizes of sewage sludge and three heating rates were employed to study the pyrolysis process. And a new method is employed to compute the pyrolysis kinetics parameters based on the TG curves.
Experimental section
Sample preparation
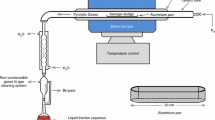
The sewage sludge obtained from the Changsha urban wastewater treatment plant (Hunan province)employing the activated sludge process for the domestic wastewater treatment was used as the studied object. And the sewage sludge has been stabilized by the addition of polyacrylamide in this plant. Before our experiment, some preparation measures were taken on the sewage sludge. The schematic of flow sheet is shown in Fig. 1.
First, the sewage sludge was dried in an oven at 105 °C for 48 h. Then, it was grounded from the original size (irregular clusters of the order of centimeters) into the size of less than 1.2 mm. By virtue of griddles, the dry sewage sludge was divided into three size ranges: d < 0.25 mm, 0.25 mm < d < 0.83 mm, and d > 0.83 mm. Then, the sample was stored in a desiccator for future use.
Sample characterization
To determine the chemical characteristics of sewage sludge, elemental and proximate analyses were carried out, and the main information was summarized in Table 1. According to the literature [17], the metal content of the dry sewage sludge was determined, and the result is shown in Table 2. The FTIR spectra (WQF-410 infrared spectrometer) were employed to analyze the original sewage sludge and residua for different size ranges at heating temperatures of 350 and 730 °C, respectively.
Pyrolysis experiment
The pyrolysis of sewage sludge was analyzed by the thermogravimetric analyzer (TGA, STA 409 model, NETZSCH, Germany) under nitrogen atmosphere. Before each run, nitrogen was introduced into the thermogravimetric analyzer to ensure an inert atmosphere, and its flow rate was kept at 100 mL/min. Then, about 10 mg sewage sludge samples were heated from 20 to 900 °C at the heating rates of 5, 20, and 35 °C/min.
Results and analysis
Thermogravimetric analysis—pyrolysis
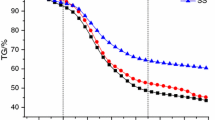
Figure 2 presents the TG and DTG of sewage sludge (d > 0.83 mm) at different heating rates. It can be seen from the DTG curves that the three stages (nearly 180–220, 220–650, and 650–780 °C) can be divided in the process of sewage sludge (d > 0.83 mm) pyrolysis. For all the samples, about 8 wt% of the total mass loss occurs in the first stage, which may be caused by the water volatilization and the decomposition of little volatile matter in the sewage sludge. In the second stage, because the dominant volatile matter of sewage sludge is decomposed, the DTG curves change sharply, although a slight shoulder peak is observed for a curve of the heating rate of 35 °C/min. This result is in a good agreement with that of Thipkhunthod et al. who reported that the sewage sludge pyrolysis mainly occurred after 200 °C and ceased at 550 °C [18]. Most of the biodegradable matter is volatized in a temperature ranging from 150 to 400 °C, and non-biodegradable organic matter volatilizes between 400 and 550 °C, which were reported by Agustín García Barneto et al. [19]. In the third stage, the DTG curves almost keep constant. The possible reason is that almost only inorganic compounds such as carbonates are left in the sewage sludge samples. These similar DTG characteristics are also found in the literature [14, 20–23].
On the other hand, it also can be seen that the higher the heating rate is, the bigger the T DTGmax (temperature associated to maximum devolatilization rate) will become. But the difference is not obvious. At the heating rates of 35 and 20 °C/min, their T DTGmax of DTG curves are nearly identical, whereas the T DTGmax of DTG curve at the heating rate of 5 °C/min is a little lower than those of the other two curves. In addition, the temperature lags should be more pronounced with the higher heating rate or when there is an obvious endothermic reaction according to Stuart A. Scott [24]. In this study, this fact is testified again, just only that there is no significant temperature lag.
Figure 3 shows the TG of sewage sludge for three sizes: d < 0.25 mm, 0.25 mm < d < 0.83 mm, and d > 0.83 mm at the heating rate of 5 (Fig. 3a), 20 (Fig. 3b), and 35 °C/min (Fig. 3c). As shown in these figures, the sewage sludge with the size of d < 0.25 mm obtains the largest total mass loss, no matter which heating rate is used, especially at the heating rate of 5 °C/min. However, the phenomenon becomes not obvious with the increase of heating rate. The reason can be explained as follows: the sewage sludge of d < 0.25 mm has more specific surface area, and hence, its heat transfer is faster and reaction is more complete. Thus, the sewage sludge of d < 0.25 mm obtains the largest total mass loss. On the other hand, the increase of heating rate can accelerate the heat transfer rate, resulting in tiny difference of the total mass loss during the heating rates 20 and 35 °C/min. Furthermore, all the TG data illustrate that the mass loss is 65 ± 5% of the total mass from room temperature to 900 °C, which corresponds to the sum of volatiles and moisture fractions of the initial analysis results in Table 1.
FTIR analysis
Figure 4 illustrates the FTIR spectra of original sewage sludge. Based on the identification of FTIR spectra listed in Table 3, the functional groups in the sewage sludge can be analyzed [22, 25–27]. However, distinguishing these chemicals by FTIR spectra is a difficult task because their functionalities are quite similar. Therefore, the FTIR analysis can be done based only on the difference in their bonds and absorption intensities in this study.
From the figure, it can be observed that there is a peak of around 3292 cm−1 between the broad band of 3100–3600 cm−1, which corresponds to OH stretching of hydroxyl groups from phenol, alcohol, and carboxylic groups, and the NH stretching of amine and amide groups according to Table 3. Simultaneously, some other bands appear in the spectra. These bands are produced by different functional groups. The broad bands around 2854 and 2924 cm−1 (peaks between 2800 and 3000 cm−1) are due to the symmetric and asymmetric stretching vibrations of aliphatic C–H bonds in the CH3 and CH2 groups. The broad bands at 1649 and 1543 cm−1 are caused by the aromatic compounds. The broad band at 1649 cm−1 is caused by C=C in aromatics structures. The broad band at 1458 cm−1 (band at 1300–1500 cm−1) is caused by aliphatic C–H deformation, and the peak at 1408 cm−1 is caused by C–H and OH of COOH in hydroxyl or amine compounds. The peaks at 1074 and 1036 cm−1 (peaks between 1000 and 1100 cm−1) are caused by C–O stretching of carbohydrate and alcohol functions, reflecting the occurrence of polysaccharides or cellulose. Combining the analysis, it can be concluded that the functional groups including NH, C–H, C=C and so on, all exist in the sewage sludge, demonstrating that the studied sludge and natural material such as lignocellulosic, cellulose, or lignin possess some comparability in component.
Figure 5 presents the FTIR spectra obtained from the three sizes of sewage sludge: d < 0.25 mm, 0.25 mm < d<0.83 mm, and d > 0.83 mm, on which the thermogravimetric runs were carried out at a heating rate 20 °C/min from 20 to 350 °C (temperature associated to maximum devolatilization rate in the second stage) (Fig. 5a) and 730 °C (temperature associated to maximum devolatilization rate in the third stage) (Fig. 5b), respectively.
As shown in Fig. 5 that the sewage sludge sizes have hardly any influence on the qualitative similarity of FTIR spectra. Comparing Fig. 5a with Fig. 4, it could be observed that some new peaks around 3695, 3618, 3375, 1701, 1616, and 1437 cm−1 occur, and some peaks around 1543, 1408, and 1232 cm−1 disappear in the FTIR spectra of sewage sludge heated to 350 °C. This phenomenon illustrates that the amino and carboxyl in the original sludge are decomposed into relatively small molecular compounds such as alcohols, ammonia, and carboxylic acid. By comparing Fig. 5b with Fig. 5a, as the sludge was heated to 730 °C, only peaks around 3427, 1529, and 1039 cm−1 occur in the FTIR spectra. It can be speculated that when the sludge was heated to 350 °C, the alcohols, ammonia, and carboxylic acid in the sludge has been mostly decomposed into gases, and only a little bit of compounds containing C–H and OH of COOH exist.
Interpretation of pyrolysis kinetics parameters
Speaking in general, the overall reaction of thermal decomposition of sewage sludge can be expressed as follows:
The reaction is supposed to be irreversible for the reason that the inert gas (N2) carries the volatile away as soon as it is formed. The best mathematical model for describing overall decomposition is independent parallel reactions [9]. The sewage sludge contains several components, each of which is assumed to decompose independently. And its kinetic equation is described as
where w i is the reacted mass fraction for reaction i at time j; m 0i the initial mass of the sample i; m ij is the sample mass for the reaction i at time j; m fi is the final mass of the sample for the reaction i; f(w i ) is the hypothetical model of the reaction mechanism or model function; n i is the order of reaction; k i is the rate constants, calculated by Arrhenius equation [9, 14]:
where A i the frequency factor for reaction i; R the gas universal constant; T is the absolute temperature; and E i is the activation energy for reaction i.
In the pyrolysis process, the heating rate β is considered to be constant and defined as,
When k i is replaced by Eq. 5, Eq. 3 can be changed into
With the help of the difference method, Eq. 7 can be converted to Eq. 8 at a very short and identical T period [28].
where, k is the sequence number of the data derived from the TG curves.
Previous researchers generally obtained the activation energy (E) and frequency factor (A) by a given reaction order [29]. However, a new method which can calculate these three parameters simultaneously was developed in this present study. Detail steps are given as follows:
By virtue of logarithm operation, Eq. 8 can be expressed as:
where w i(k+1), w ik , T i(k+1), and T ik can be obtained from the TG data. Defined
Thus, Eq. 9 can be written as:
Several data sets of x, y, z were first calculated with the TG data. Then, by fitting these data sets with the surface fitting tool in software MATLAB7.11.0 (R2010b), the pyrolysis kinetics parameters (n, E, and A) can be obtained. Meanwhile, other parameters such as R-square and RMSE (root mean square error) reflecting the precision of fitting will be shown in the surface fitting tool.
Considering that mostly volatile matters are decomposed in the second stage, only the kinetic parameters of this stage is interpreted in this section. The results are shown in Tables 4 and 5 according the above method. Figure 6 gives the plane fitting graph of data sets of x, y, z with the TG data of sewage sludge (d > 0.83 mm) at 20 °C/min and its residuals plot is shown in Fig. 7. As shown in the figures that the experimental points are almost in the fitting plane and each point’s residual is small, illustrates that the calculation method is effective to the experimental data fitting.
From Table 4, it can be seen that the heating rate has a little effect on the order of pyrolysis reaction and the order was in the range of 1.6–1.8. However, for the average activation energy of pyrolysis reaction, there was some fluctuation with the variation of heating rates. The results agree with Conesa et al. [11] who reported that the activation energy was in the range of 17–332 kJ/mol. As shown in Table 5 that with the increase of the sewage sludge size, the pyrolysis reaction order increases continuously while the activation energy almost keeps a constant. Furthermore, it can be calculated from Tables 4 and 5 that the biggest frequency factor is 7.7 × 104, lower than the range of 1010 s−1 and in line with Vamvuka et al. [30]. Meanwhile, the frequency factor increases with the increase of heating rate and sewage sludge size, which was agreed with Jingai Shao et al. [31].
Conclusions
Three sizes of sewage sludge samples were pyrolyzed with TGA at three heating rates. Elemental and proximate analyses were employed to study their pyrolysis characteristics. FTIR analysis was employed to determine the functional groups of sewage sludge. By virtue of a new method, the kinetic parameters of sewage sludge pyrolysis were calculated and discussed. The following items were concluded:
-
(1)
By means of the thermogravimetric analysis, the mass loss is about 65 ± 5 wt% of the total sludge mass at all experiments. In general, the whole TG curve is divided into three stages, which are nearly 180–220, 220–650, and 650–780 °C, respectively. The sewage sludge of d < 0.25 mm obtains the largest total mass loss, no matter which heating rate is used. The phenomenon is especially more obvious at the heating rate of 5 °C/min than that of the heating rate of 20 and 35 °C/min. There is no significant temperature lag in the thermogravimetric analysis. With the increase of heating rate, the T DTGmax rises.
-
(2)
FTIR analysis illustrates that the functional groups including NH, C–H, C=C and so on, all exist in the sewage sludge, demonstrating that the studied sludge and natural material such as lignocellulosic, cellulose, or lignin possess some comparability in the component. In the second stage, the alcohols, ammonia, and carboxylic acid in the sludge have been mostly decomposed into gases, and only a little bit of compounds containing CH and OH of COOH exist.
-
(3)
Fitting method is applied to determine the kinetics parameters of the second stage of sewage sludge pyrolysis. The reaction order is in the range of 1.6–1.8 and the activation energy is nearly 45 kJ/mol. The frequency factor increases with the increase of heating rate and sewage sludge size. All are in the range of what has been reported. It will be observed that no clear tendency may be established of the evolution of the reaction order and the activation energy with the increase of sewage sludge size and heating rate.
References
Rio S, Faur-Brasquet L, Coq L, Cloirec LP. Structure characterization and adsorption properties of pyrolized sewage sludge. Environ Sci Technol. 2005;39(11):4249–57.
Campostrini R, Ischia M, Palmisano L. Pyrolysis study of sewage sludge by TG-MS and TG-GC-MS coupled analyses. J Therm Anal Calorim. 2007;87:567–74.
Viana MM, Melchert MBM, Morais LC, Buchler PM. Sewage sludge coke estimation using thermal analysis. J Therm Anal Calorim. 2011;10:1392–401.
Capana AS, Martins QV, Crespi MS, Ribeiro CA, Barud HS. Thermal behavior of residues (sludge) originated from Araraquara water and sewage treatment station. J Therm Anal Calorim. 2009;97:601–4.
Ninan KN, Krishnan K, Krishnamurthy VN. Kinetics and mechanism of thermal decomposition of insitu generated calcium carbonate. J Therm Anal Calorim. 1991;37:1533–43.
Campanella L, Tomassetti M, Tomellini R. Thermoanalysis of ancient, fresh and waterlogged woods. J Therm Anal Calorim. 1991;37:1923–32.
Jaber JO, Probert SD. Pyrolysis and gasification kinetics of Jordanian oil-shales. Appl Energy. 1999;63:269–86.
Ebeling JM, Jenkins HM. Physical and chemical proper- ties of biomass fuels. Trans ASAE. 1985;28:898–902.
Sorum L, Grønli MG, Hustad JE. Pyrolysis characteristics and kinetics of municipal solid wastes. Fuel. 2001;80:1217–27.
Conesa JA, Font R, Fullana A, Caballero JA. Pyrolysis of polyethylene in a fluidized bed reactor. Fuel. 1998;77:1469–75.
Conesa JA, Marcilla A, Prats D, Rodriguez M. Kinetic study of the pyrolysis of sewage sludge. Waste Manag Res. 1997;15:293–305.
He P, Shao L, Gu G, Li G. Mechanism and kinetics of low-temperature thermo-chemical conversion process of sewage sludge. Water Sci Technol. 2001;44:341–7.
Stolarek P, Ledakowicz S. Thermal processing of sewage sludge by drying, pyrolysis, gasification and combustion. Water Sci Technol. 2001;44(10):333–9.
Calvo LF, Otero M, Jenkins BM. Heating process characteristics and kinetics of sewage sludge in different atmospheres. Thermochim Acta. 2004;409:127–35.
Nowicki L, Antecka A, Bedyk T. The kinetics of gasification of char derived from sewage sludge. J Therm Anal Calorim. 2010;12:1032–41.
Othman MR, Park YH. Thermogravimetric characteristics and pyrolysis kinetics of Giheung Respia sewage sludge. Korean J Chem Eng. 2010;27(1):163–7.
Zhai YB, Liu Q, Zeng GM. Experimental study on the characteristics of sewage sludge pyrolysis under the low temperature conditions. Environ Eng Sci. 2008;25(8):1203–12.
Thipkhunthod P, Meeyoo V. Describing sewage sludge pyrolysis kinetics by a combination of biomass fractions decomposition. Appl Pyrolysis. 2007;79:78–85.
Barneto AG, Carmona JA. Kinetic models based in biomass components for the combustion and pyrolysis of sewage sludge and its compost. Appl Pyrolysis. 2009;86:108–14.
Caballero JA, Font R, Marcilla A, Conesa JA. Thermogravimetric studies on the thermal decomposition of polyethylene. J Anal Appl Pyrolysis. 1997;40–41:433–50.
Chen X, Jeyaseelan SJ. Study of sewage sludge pyrolysis mechanism and mathematical modeling. Environ Eng. 2001;127(7):585–93.
Mansuy L, Bourezgui Y, Garnier-Zarli E, Jarde E, Révéille V. Characterization of humic substances in highly polluted river sediments by pyrolysis methylation-gas chromatography–mass spectrometry. Org Geochem. 2001;32:223–31.
Urban DL, Antal JM. Study of the kinetics of sewage sludge pyrolysis using DSC and TGA. Fuel. 1982;61(9):799–806.
Stuart AS, John SD, John FD, Allan NH. Thermogravimetric measurements of the kinetics of pyrolysis of dried sewage sludge. Fuel. 2006;85:1248–53.
Réveillé V, Mansuy L, Jardé E, Garnier-Zarli E. Characterization of sewage sludge-derived organic matter: lipids and humic acid. Org. Geochem. 2003;34:615–27.
Sharma RK, Wooten JB, Baliga VL, Lin X, Chan WG, Hajaligol MR. Characterization of chars from pyrolysis of lignin. Fuel. 2004;83:1469–82.
Amir S, Hafidi M, Merlina G, Revel JC. Structural characterization of fulvic acids during composting of sewage sludge. Process Biochem. 2005;40:1693–700.
Ji AM, Zhang S, Lu XB, Liu Y. A new method for evaluating the sewage sludge pyrolysis kinetics. Waste Manag. 2010;30:1225–9.
Jiang JG, Du XJ, Yang SH. Analysis of the combustion of sewage sludge-derived fuel by a thermogravimetric method in China. Waste Manag. 2010;30:1407–13.
Vamvuka D, Kakaras E, Kastanaki E, Grammelis P. Pyrolysis characteristics and kinetics of biomass residuals mixtures with lignite. Fuel. 2003;82:1949–60.
Shao JG, Yan R, Chen HP. Pyrolysis characteristics and kinetics of sewage sludge by thermogravimetry Fourier transform infrared analysis. Energy Fuel. 2008;22:38–45.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (50908080), the Fundamental Research Funds for the Central Universities, and the Doctoral Fund of Ministry of Education of China (Grant No. 20090161120042).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhai, Y., Peng, W., Zeng, G. et al. Pyrolysis characteristics and kinetics of sewage sludge for different sizes and heating rates. J Therm Anal Calorim 107, 1015–1022 (2012). https://doi.org/10.1007/s10973-011-1644-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-011-1644-0