Abstract
The effect of three flame retardants, K2CO3, Na2SiO3·9H2O, and Na2B4O7·10H2O on the process and composition of volatile products of the thermal degradation of wood has been investigated by the thermogravimetric (TG), differential thermogravimetry (DTG), differential thermal analysis (DTA), and the synchronous thermogravimetry–mass spectrometry (TG–MS) analysis methods. The results showed that the ion current intensity and ion peak area of m/z = 18 and 44 MS signals were increased by the flame retardants but the ion peak area of m/z = 28 MS signal was decreased (except K2CO3) at the meantime. What’s more, the ion current intensity and ion peak area of m/z = 60 and 68 MS signals were also decreased (except K2CO3), which mean that Na2B4O7 can significantly enhances the dehydration and inhibits the depolymerization of wood. Although K2CO3 accelerates the dehydration reaction, it cannot inhibit the depolymerization reaction effectively, so the flame retardant efficiency of K2CO3 is decreased with the higher concentration. The catalysis of dehydration reaction of Na2SiO3 is the worst one.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
At present, there is growing interest to use wood and wood-based materials for applications in both residential and non-residential building construction [1, 2]. However, wood and wood-based materials frequently implicate in fire due to their inflammable structural, which would cause injuries and fatalities. To avoid fire, many methods are employed to treat the wood and wood-based materials, among which the use of flame-retardant treatment would be the most promising one.
Boron compounds are often considered as nice fire retardants for wood because of their excellent properties, such as preservative effectiveness, neutral pH, and less impact on mechanical properties than some other flame retardant chemicals [3–5]. B2O3 alkaline salts may melt at relatively low temperature and decompose to B2O3 and form a glassy protective surface layer at 325 °C, which melts at 500 °C and successfully used for cellulose-based products [6]. Another glass-forming inorganic additive commonly used for wood is sodium silicate [7]. These glass-forming inorganic additives make it possible to form a protective glassy layer from components at ignition. The glassy layer acts as a thermal insulator, raising the initial degradation temperature (IDT) of wood. Likewise, the layer also serves as a barrier for oxygen, as evidenced from a significant shift of exothermic peaks attributed to oxygen combustion to higher temperatures, which is beneficial to the improvement of flame retardancy [8]. It is different from the forenamed flame retardants; potassium carbonate will not melt or decompose under 800 °C, but it also has a high flame retardant efficiency for wood materials [9, 10].
Although such systems have been examined in a number of studies, the optimization of the composition and the mechanism has not been completed. What’s more, no detailed quantitative information is available about the product yields and composition for wood treated with borax, sodium silicate, or potassium carbonate.
Various methods have been developed for evaluating the performance of flame retardants, such as thermal analysis, tunnel flame-spread tests, critical oxygen index tests, smoke production tests, cone calorimeter, and analysis of solid residue or gaseous products of thermal decomposition [11–15]. Thermal analysis is a simple, convenient, fast and effective method for the study of pyrolysis and flame retardants [16]. Especially, the thermogravimetry–mass spectrometry (TG–MS) analysis is a very usefully method to confirm the content and species of the gaseous products of thermal decomposition and to contribute to a better understanding of the mechanisms of flame retardant, which might facilitate the development of new flame retardant products.
In this study, wood was treated with three chemical substances, potassium carbonate, borax, and sodium silicate to impart flame retardancy. For a study of flame retardancy from the standpoint of thermal degradation, the samples were subjected to thermogravimetric (TG) analysis, differential thermogravimetry (DTG), and differential thermal analysis (DTA) to determine if there were any characteristic correlations between thermal degradation behavior and the level of flame retardancy. The effects of flame retardants on the main gaseous products of the thermal decomposition were analyzed by the thermogravimetry–mass spectrometry analysis, and the morphology of the char residue was also observed by a scanning electron microscopy.
Experimental
Materials
The wood specimens (150 × 6 × 3 mm) were obtained from the sapwood portions of eugene poplar (Hebei Province, China) and were used for the limiting oxygen index (LOI) experiments. For this purpose, the samples were immersed in distilled water at 90 °C for 2 h, and then thoroughly rinsed with distilled water and left to oven-dried at 70 °C for 24 h. All of the K2CO3, Na2SiO3·9H2O, and Na2B4O7·10H2O used were of analytical grade (>99.0%) from Tianjin Chemical-Reagents Corp. in PR China.
Flame-retarding treatment of wood samples
The reagents of K2CO3, Na2SiO3·9H2O, and Na2B4O7·10H2O were dissolved in distilled water, with the mass concentration of 1, 5, 10, and 20%, respectively, for preparing the flame retardant wood. The purified wood specimens (150 × 6 × 3 mm) were immersed in the four aqueous solutions (the mass concentration is 1, 5, 10, and 20%, respectively) of K2CO3, Na2SiO3·9H2O, and Na2B4O7·10H2O, respectively, at 80 °C for 2 h, and then oven-dried at 70 °C for 24 h. The samples were stored in desiccators until the test.
The mass gains (WG) of the flame retardant specimens were calculated from the following equation:
where W 0 is the oven-dried mass (g) of a wood specimen before impregnation and W 1 is the final oven-dried mass of the treated wood specimen.
Limiting oxygen index method
The LOI is the minimum percentage oxygen for maintaining specimen’s flaming combustion under specified laboratory conditions. LOI values were determined with General Model HC-2 LOI instrument (Nanjing Jiangning Analysis Instrument Factory, Nanjing, China) accordance with ASTM D2863-2000.
Thermal analysis
Thermal degradation of the samples was determined by TG, DTG, and DTA. The analyses were carried out on a WCT-2 (Beijing optical instrument Co. Ltd., China) thermoanalytical apparatus. 7 ± 0.2 mg samples (platinum pan) were heated from ambient temperature to 800 °C at a heating rate of 20 °C min−1 under air with a flowing rate of 60 mL min−1.
Scanning electron microscopy analysis
First, the wood samples were placed in a muffle furnace in high pure nitrogen at 350 °C for 10 min, and then were cooled to ambient temperature. The morphology of the char formed in this way was investigated by means of a SEM-KYKY-2800B scanning electron microscopy (Chinese Academic of Science Instrument Factory, Beijing, China). The surfaces of the char were coated with gold prior to analysis.
Thermogravimetry–mass spectrometry analysis
A TG–MS made by Netzsch Co. Ltd. (STA 449 C-QMS 403 C) was used to analyze the fragmentates from the thermobalance. The TG was performed in high pure argon (99.999%), and the following rate is 25 mL min−1. Approximately 7 mg sample was heated at 10 °C min−1 from ambient temperature up to 800 °C in the dynamic experiments. The mass spectrometry analysis with an ion source of electron impact at 70 eV electron energy scan from mass 10 to 160 with a speed of 0.2 s for each mass unit. The connection between the thermobalance and the mass spectrometer was done by means of a quartzose capillary, maintained at 200 °C.
Results and discussion
The WG of the flame retardant specimens
As shown in Fig. 1, the WG% of the wood samples treated with different flame retardants are increased with the increase of the concentration of the flame retardants solutions. When the mass concentration is 1%, the WG% is negative value; especially the Na2SiO3-treated sample, the WG% is −5.42%, the reason is that there are some soluble substance can be dissolved by the flame retardants solutions. If we deduct the influence of the soluble substance, the WG% of the wood samples almost at the same level when treated with different flame retardants.
Limiting oxygen index
The LOI results are shown in Fig. 2. The results show that the LOI values of the treated samples increase with the concentration of the flame retardants solutions. The LOI values of the Na2B4O7-treated samples present a linear increase with the solution concentration, while the ones of the Na2SiO3-treated samples increase inconspicuously. When the mass concentration increased from 1 to 20%, the LOI value increases from 25.1 to 30.1%, then only five units were increased. When the mass concentration is <10%, K2CO3 shows the highest flame retardant efficiency, the LOI values increase from 20.8 to 35.5%. About the Na2B4O7 solution treated samples, when the mass concentration is increased to 20%, the LOI values increase from 20.8 to 38.9%. In other words, the flame retardancy of wood is improved significantly.
In order to compare the effect of the three flame retardants on the fire retardant and thermal properties of wood, the wood samples treated with three solutions (with the identical mass concentration of 5%) have been investigated by the thermal analysis, TG–MS, and SEM.
Thermal analysis
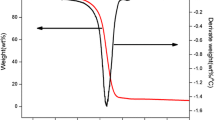
The representative associated TG, DTG, and DTA curves of the purified wood and the Na2B4O7 (5%) treated sample are shown in Figs. 3, 4. The related experiments were carried out in dynamic air atmosphere from ambient temperature to 800 °C.
In Figs. 3, 4, it can be seen that the TG and DTG curves of the thermal degradation process appear to be divided into four mass loss stages. At the first stage, from ambient temperature to 100 °C, the mass loss is resulted from the removal of water. Among the samples, the mass loss of the K2CO3 treated ones is the highest, which indicates that the hygroscopic property of K2CO3 is noticeable.
The second stage, from about 200 to 350 °C, corresponds mainly to hemicellulose and cellulose decomposing into char residues and CO2, CO, CH4, CH3OH, and CH3COOH, etc. As temperature increase gradually, the mass loss on the TG curve accelerates [17]. It is also reported that the second stage in the thermal decomposition of samples plays a key role attributed to the combustibility [18]. The results of the TG and DTG (Tables 1 and 2) show that, at this stage, the temperature of the maximum rate of mass loss (T peak) of the treated wood is lower than the untreated wood. In particular, the mass loss and the maximum rate of mass loss (R peak) at this stage are decreased obviously. As a result, the char residues which are directly related to the flammability properties are increased by of flame retardants.
The flame retardant efficiency of the three flame retardants is different, and this result can be related to the thermal degradation behavior of the treated wood samples in the second stage. The IDT of untreated wood sample is 245 °C, which was improved to 253 °C after treated by Na2B4O7. The mass loss and the R peak at this (the second) stage were decreased and the yield of the carbonized residue was increased and these results can be attributed to the fact that Na2B4O7 melts at 325 °C and decomposes to B2O3 and forms a glassy protective surface layer. Hence, the decomposition temperature was increased significantly. It is very different from the above-mentioned result; the IDT of the sample decreased from 245 to 202 °C after treated by K2CO3, while the LOI value indicates that the flame retardancy of the sample was improved significantly. These results indicate that the K2CO3 practice a different flame retardant mode of action to wood and K2CO3 catalyzed the degradation reaction of the wood in the second stage. The IDT of the sample was also decreased by 20 °C after treated with Na2SiO3, but it is not as obvious as the K2CO3 treated one. What’s more, Na2SiO3 and Na2B4O7 are quite different, and it cannot be melt at such a temperature range, so the flame retardancy of the Na2SiO3-treated sample is worst.
After the second stage, mass loss of the residual materials of the purified wood are found to be slow in the temperature range 353–428 °C. The TG curve shows a mass loss of 13.8%. At this (third) stage, the less stable aliphatic groups are preferentially decomposed through homolytic cleavage of C–C and C–H bonds, and the resultant product is a highly condensed and cross-linked carbonaceous materials. The obvious difference between the treated and untreated wood samples is that the mass losses and the final decomposition temperature (FDT) at this stage were increased of the treated samples simultaneously. These results mean that the stability and amount of the char residue of the treated samples were improved, which is beneficial for the flame retardancy of wood.
The fourth stage, the temperature range of 428–679 °C, is related to the oxidation of char residue. In this stage, the TG curve of untreated sample shows a mass loss of 12.3%. While for the Na2B4O7-treated sample, the char residue that formed in this degradation stage increased by 6% compared with the untreated one. In this stage, the IDT, the FDT, and the T peak of the flame retardant treated samples increased significantly compared to those of the untreated one’s, while the R peak was decreased obviously. Especially the sample which treated with K2CO3, the IDT of the sample, was raised from 428 to 558 °C, and the R peak at this stage was decreased from 1.138 to 0.186 mg min−1. All of these results indicate that the stability of the char residue was improved, and which are advantageous to improve the flame retardancy of wood.
As shown in Figs. 3, 4 and Table 2, there are two exothermic peaks on the DTA curve of the untreated sample. The first one at 350 °C is attributed to flaming combustion of volatile products, and the second one at about 440 °C is attributed to glowing combustion of the char residual [2]. What’s more, there is great difference between the DTA curve of the untreated sample and that of the ones treated with Na2B4O7. After the first sharp exothermal peak changed to be relatively smooth, and the second peak has been uplifted. These results mean that the combustion of the wood tends to be much steadier at the second stage, and the heat release rate was slow down after treated by the flame retardants. What’s more, the amount of combustible volatiles was decreased while the amount of residue char increased at this stage. Especially for the samples treated with Na2SiO3 and K2CO3, there are three exothermic peaks on the DTA curve. The first exothermic peak temperature (T E1) was decreased from 355 to 310 °C and 297 °C, respectively, and the second exothermic peak of the untreated sample was split into two independent peaks after treated by the flame retardants. The second (T E2) and the third (T E3) peak temperatures (464 and 606 °C) of the sample treated with K2CO3 are higher than the second exothermic peak temperature (443 °C) of the untreated sample. These results mean that Na2SiO3 and K2CO3 had changed the degradation reaction of wood, contributing to the enhancement of the amount and stability of the char residue.
To verify these results, the morphology of the char residue formed after combustion of the treated and untreated wood samples were observed through SEM. As shown in Figs. 5, 6, 7, and 8, the char residues of the treated and untreated wood samples are quite different. The morphology of the char residue generated from the untreated wood shows an incompact structure with gray color, while those generated from the treated ones show a more condensed structure, especially the char residue generated from the Na2B4O7-treated sample, on whose surface melted object can be observed. There is no obvious melted object on the surface of the char residue of the sample treated with Na2SiO3, which means that Na2SiO3 cannot be melted at this temperature. The condensed structure can form a barrier to inhibit combustible gases and transfer heat energy to the wood bulk, which is beneficial to the improvement of flame retardancy.
TG–MS analysis
The TG results of the samples (5%) that performed in argon were shown in Fig. 9. As shown in Fig. 9, there is only one degradation stage (from 220 to 400 °C) on the TG curves of the samples, and the TG curve of the sample treated with Na2SiO3 is very similar to that of K2CO3 treated ones. The mass loss amount and the IDT of the treated samples were lower than those of the untreated samples, which is accordant with the results in air.
It is known that the thermal degradation of cellulose usually takes place during the temperature range of 250–400 °C through two competing pathways [11]: one is the dehydration which leads to char and gases (mainly, CO, CO2, and H2O) and the other is the depolymerization which leads to tar and volatiles through the formation of levoglucosan.
In Figs. 10, 11, the representative signals of m/z = 28 and 44 MS samples are shown in ampere, respectively. As shown in Figs. 10, 11 and Table 3, corresponding with the TG curves, the ion peak temperature of the sample treated with flame retardant was decreased obviously (except the m/z = 28 MS signal of wood treated with Na2B4O7). For the wood treated with K2CO3 and Na2B4O7, the ion current intensity and the ion peak area of m/z = 18 that attributed to water [19] was enhanced obviously comparing with those of the untreated ones, which mean that the water output of the K2CO3 and Na2B4O7 was increased. Although the ion current intensity (m/z = 18) of the sample treated with Na2SiO3 was increased from 84.5 to 182.4, the ion peak area was decreased appreciably, indicating that the catalysis of Na2SiO3 to the dehydration reaction of wood is inconspicuous.
For the MS signal of m/z = 44 (Fig. 11), which is mainly attributed to carbon dioxide, both the ion peak area and the ion current intensity of all the treated samples were increased, which indicated that the carbon dioxide output of the treated samples was increased. While the increase of the carbon dioxide output of the sample treated with Na2SiO3 is limited, so the flame retardancy of Na2SiO3 for wood is faintish. The MS signal of m/z = 44 intensified again after 600 °C for the sample treated with Na2B4O7, indicating that the char residue of the sample treated with Na2B4O7 can decompose further after 600 °C.
The MS signal of m/z = 28 (Fig. 10) is mainly due to CO and to a lesser extent aliphatic molecules in the pyrolysis of cellulosic materials. The ion current intensity and the ion peak area (m/z = 28) of the sample treated with Na2B4O7 or Na2SiO3 were decreased, which indicated that the CO output of the treated samples was decreased and the decrease of the CO output of the sample treated with Na2SiO3 was also limited. The ion peak area (m/z = 28) of the sample treated with K2CO3 was increased obviously, which means that the degradation mechanism of the wood treated with K2CO3 has been changed.
The ion of m/z = 60 is an indicator for levoglucosan [19, 20]. As shown in Table 3, both the ion current intensity and the ion peak area values of m/z = 60 were decreased for the decomposition of wood in the presence of Na2B4O7 and Na2SiO3. In addition, m/z = 68 is mainly attributed to levoglucosenone and can be regarded as a representative of levoglucosenone [19, 20]. In this work, there is a very small quantity of m/z = 68, the MS signals of which showed only some discontinuous points, whose ion peak area was meaningless. Hence, only the maximum ion current intensities of the samples are shown in Table 3. The samples treated with Na2B4O7 and Na2SiO3 appear with a relative decrease of the ion current intensity of m/z = 68 MS signal, which means that a lower level of levoglucosenone was recorded in the presence of the flame retardants. Compared with the untreated wood sample, the ion current intensity and the ion peak area values of m/z = 60 of the sample treated with K2CO3 were at the same level, which means that K2CO3 can catalyze the dehydration effectively and at the same time it did not inhibit the depolymerization reaction of wood. We can conclude that K2CO3 can catalyze the dehydration effectively but do not inhibit the depolymerization reaction of wood. That would be the reason why the flame retardancy of wood was not improved as anticipated when the concentration of K2CO3 solution increasing at a relatively high value.
Conclusions
Na2B4O7 is a very effective flame retardant to wood, which would melt at a relatively low temperature and then form a protective glassy B2O3 layer over the surface of wood, leading to an enhancement of the IDT. What’s more, Na2B4O7 would catalyze the dehydration reactions of wood. As a result, the output of water and CO2 would be increased obviously and the depolymerization reaction of wood would be inhibited effectively. Meanwhile, an intermediate carbonized product was formed with a more condensed structure and the content of flammable gas among the volatile products would be decreased significantly. All of the above facts would be responsible for the enhancement of the flame retardancy of the sample. Although K2CO3 would catalyze the dehydration reaction of wood to increase the output of water and CO2, the output of CO, which would contribute to the combustion of the sample, would be increased simultaneously. What’s more, K2CO3 cannot inhibit the depolymerization reaction of wood effectively, especially in relatively high concentration. As a result, K2CO3 shows a higher flame retardant efficiency in relatively low concentration; when the concentration of K2CO3 is raised, its promotion effect to the depolymerization reaction of wood would be enhanced, leading to the decrease of the flame retardant efficiency. Among the three kinds of flame retardants, Na2SiO3 possesses the lowest catalysis and cannot melt to form a protective layer at a relatively low temperature as Na2B4O7 does. Hence, there is no obvious increase for the output of water and CO2 when the sample treated with Na2SiO3 degrades comparing with the degradation process of the untreated wood sample. And Na2SiO3 shows the lowest flame retardant efficiency.
References
Gao M, Sun CY, Wang CX. Thermal degradation of wood treated with flame retardants. J Therm Anal Calorim. 2006;85(3):765–9.
Ondrej G, Franck P, Desana M, Helena M, Andrea B. Intumescence in fire retardancy of lignocellulosic panels. Polym Degrad Stab. 2003;82:373–7.
Ergun B, Mustafa KY, Mustafa A, Abdullah S, Hseyin P, Mehmet C. Some physical, biological, mechanical, and fire properties of wood polymer composite (WPC) pretreated with boric acid and borax mixture. Construct Build Mater. 2007;21:1879–85.
Yalinkilic MK, Takahashi M, Imamura Y, Gezer ED, Demirci Z, Ilhan R. Boron addition to non or low formaldehyde cross-linking reagents to enhance biological resistance and dimensional stability for wood. Holz als Roh Werkstoff. 1991;57(1):151–63.
Chen PYS, Puttmann ME, Williams LH, Stokke DD. Treatment of hardwood lumber with borate preservation. For Prod J. 1997;47(6):63–8.
Marosi G, Márton A, Anna P, Bertalan G, Marosfoi B, Szép A. Ceramic precursor in flame retardant systems. Polym Degrad Stab. 2002;77:259–65.
Enyu X, Minxiu Z. Flame retardant science and application. 1st ed. Beijing: National Defence Industry Press; 1988.
Silvo H, Majda SS, Karin SK, Marjan B, Janez J, Miran G. Flame retardant activity of SiO2-coated regenerated cellulose fibres. Polym Degrad Stab. 2007;92:1957–65.
Kadir O, Abdullah CI, Erol B, Salih A. The effect of potassium carbonate borax and wolmanit on the burning characteristics of oriented strandboard (OSB). Construct Build Mater. 2007;21:1457–62.
Dobele G, Urbanovich I, Zhurins A, Kampars V, Meier D. Application of analytical pyrolysis for wood fire protection control. J Anal Appl Pyrolysis. 2007;79:47–51.
Liodakis S, Bakirtzis D, Dimitrakopoulos AP. Autoignition and thermogravimetric analysis of forest species treated with fire retardants. Thermochim Acta. 2003;399:31–42.
Franceschi E, Cascone I, Nole D. Thermal, XRD and spectrophotometric study on artificially degraded woods. J Therm Anal Calorim. 2008;91(1):119–25.
Xu Q, Griffin GJ, Jiang Y, Preston C, Bicknell AD, Bradbury GP, White N. Study of burning behavior of small scale wood crib with cone calorimeter. J Therm Anal Calorim. 2008;91(3):787–90.
Streibel T, Geißler R, Saraji-Bozorgzad M, Sklorz M, Kaisersberger E, Denner T, Zimmermann R. Evolved gas analysis (EGA) in TG and DSC with single photonionisation mass spectrometry (SPI-MS): molecular organic signatures from pyrolysis of soft and hard wood, coal, crude oil and ABS polymer. J Therm Anal Calorim. 2009;96:795–804.
Tzamtzis N, Liodakis S, Pappa A, Statheropoulos M, Parissakis G. The effect of (NH4)2HPO4 and (NH4)2SO4 on the composition of volatile organic pyrolysis products of cellulose: PY-GC studies. Polym Degrad Stab. 1997;56:287–90.
Qu HQ, Wu WH, Jiao YH, Xu JZ. Thermal behavior and flame retardancy of flexible PVC treated with Al(OH)3 and ZnO. Polym Int. 2005;54:1469–73.
Liodakis S, Bakirtzis D, Dimitrakopoulos A. Ignition characteristics of forest species in relation to thermal analysis data. Thermochim Acta. 2002;390(1–2):83–91.
Gao M, Ling BC, Yang SS, Zhao M. Flame retardance of wood treated with guanidine compounds characterized by thermal degradation behavior. J Anal Appl Pyrolysis. 2005;73:151–6.
Qingfeng L, Chunxiang L, Yonggang Y, Fu H, Licheng L. Investigation on the effects of fire retardants on the thermal decomposition of wood-derived rayon fiber in an inert atmosphere by thermogravimetry–mass spectrometry. Thermochim Acta. 2004;419:205–9.
Pappa A, Mikedi K, Tzamtzis N, Statheropoulos M. Chemometric methods for studying the effects of chemicals on cellulose pyrolysis by thermogravimetry–mass spectrometry. J Anal Appl Pyrolysis. 2003;67(2):221–35.
Acknowledgements
The authors gratefully acknowledge the financially supported by the Natural Science Fund of Hebei Province (China No. B2007000144) and the science and technology study direction plan of Baoding city (China No. 09ZF020).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Qu, H., Wu, W., Wu, H. et al. Study on the effects of flame retardants on the thermal decomposition of wood by TG–MS. J Therm Anal Calorim 103, 935–942 (2011). https://doi.org/10.1007/s10973-010-1103-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-010-1103-3