Abstract
A novel silicon-containing trifunctional cycloaliphatic epoxide resin tri(3,4-epoxycyclohexylmethyloxy) phenyl silane (TEMPS) was synthesized and characterized by FTIR, 1H NMR, 13C NMR, and 29Si NMR spectroscopic analysis. A series of flame-retardant formulations by blending TEMPS with a commercial epoxide resin DGEBA (EP828) in different ratios were prepared, and exposed to a medium pressure lamp to form the cured films in the presence of diaryliodonium hexafluorophosphate salt as a cationic photoinitiator. The thermal degradation behaviors of the cured films were evaluated by thermogravimetric analysis. The char yields under nitrogen and air atmospheres increased along with the TEMPS content. The limiting oxygen index (LOI) value increased from 22 for EP828 to 30 for TEMPS80, demonstrating the improved flame retardancy. The data from the dynamic mechanical thermal analysis showed that TEMPS had good miscibility with EP828. The T s and T g both decreased from 93 and 138 to 78 and 118 °C, respectively. The crosslinking density (ν e) increased along with the TEMPS content. The mechanical property measurements indicated that the addition of TEMPS led to a decrease in the tensile strength and an increase in the elongation-at-break.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Since the introduction in the 1940s, epoxy resins have been widely used on a large scale for adhesives, coatings, castings, and advanced composites in aerospace and electronic industries due to their excellent thermal and chemical resistance, superior mechanical properties, and high adhesion to many substrates [1–3]. However, to meet some application requirements, the flammability of epoxy resins is a major disadvantage in the use. Moreover, most thermo-curing epoxy resins are used as mutually reactive component mixtures. Owing to their poor handling characteristics, epoxy resins cannot be easily used in automated assembly-line processes. Therefore, it is worth to develop one-component, shelf-stable flame-retardant epoxy resins.
As an alternative to halogenated flame retardants, which release toxic gases and corrosive smoke during combustion [4], silicon-containing flame retardants have been considered environmental friendly because it can reduce the harmful impact on the environment compared with the existing materials. Two approaches for preparing epoxy resins with silicon covalently bonded to the final epoxy network have been reported. One is to synthesize the silicon-containing epoxides, which can be thermo-cured on their own or blended with other epoxy comonomers in the presence of curing agents [5–11]. Another is to synthesize diamino-terminated siloxanes, which can be used as curing agents to effectively introduce silicon into thermo-curing epoxy resins [6, 11, 12].
However, apart from thermally curing systems, UV-curing systems, which can be classified into free-radical and cationic systems, have been successfully introduced into many applications, such as printing inks, electronics, activating adhesives, and coatings [13, 14]. Although many interests have been focused on free-radical systems due to their high photopolymerization activity, the less utilized cationic curing, based on the photo-generation of acid and consecutive cationic polymerization, still presents a number of advantages. First, the epoxide resins used in cationic photopolymerization systems are characterized by being less toxic and irritant with respect to acrylates largely used in radical photopolymerization. Second, the cationic photopolymerization is not inhibited by oxygen, whereas the free-radical photopolymerization is usually inhibited by oxygen and even sometimes must be carried out in inert atmosphere [15, 16]. Third, there is low shrinkage during UV-curing of cationic curable materials, thereby resulting in good adhesion to substrates. More importantly, the cationic systems containing epoxide resins are usually one-component formulations and possess good shelf stability at room temperature in dark, compared with two-component mixtures used in thermo-curing systems.
There has been little work performed to prepare silicon-containing epoxide resins as flame-retardant components used for cationically UV-curing systems. In this study, we synthesized a trifunctional silicon-containing cycloaliphatic epoxide, tri(3,4-epoxycyclohexylmethyloxy) phenyl silane (TEMPS), used as a new reactive-type flame retardant. TEMPS monomer has good miscibility with different ratios of commercial DGEBA resin (EP828). The flame retardancy, thermal and mechanical properties, and the dynamic mechanical thermal behavior were investigated in detail.
Experimental
Materials
Phenyltrimethoxyl silane and titanium tetraisopropoxide were supplied by Fluka. 3-Cyclohexene-1-carboxaldehyde and m-chloroperoxybenzoic acid (mCPBA) were purchased from Aldrich Chemical Co. NaBH4, Na2SO4, NaHCO3, Na2S2O3, tartaric acid, methanol, and toluene were purchased from the First Reagent Co. of Shanghai, China. DGEBA resin (EP828) was obtained from Shell Chemical Co. USA. Diaryliodonium hexafluorophosphate salt (Irgacure 250) was supplied as a gift by BASF (Ciba-Geigy) and used as a cationic photoinitiator.
Synthesis
Synthesis of tri(cyclohex-3-enylmethoxy) phenyl silane (TCMPS)
3-Cyclohexenyl-l-methanol (CM) was prepared in a high yield by the reduction of 3-cyclohexene-1-carboxaldehyde with NaBH4 in methanol according to the literature reported elsewhere [17].
Phenyltrimethoxy silane (9.92 g; 0.050 mol), CM (33.65 g; 0.30 mol), and titanium tetraisopropoxide (0.30 g; 0.001 mol) were added into a 250-mL flask, and stirred at 60 °C in vacuo to remove the formed methanol. The progress of the reaction was monitored by 1H NMR analysis until complete disappearance of the peak for Si–O–Me group. The unreacted CM was removed under reduced pressure at higher temperature. Then the reaction mixture was washed twice with 5 wt% tartaric acid, three times with 5 wt% NaHCO3, and then with water and brine. The organic layer was dried over Na2SO4 and filtered. Then the solvent was evaporated under vacuum, obtaining a liquid product with a yield of 86%, named TCMPS.
1H NMR (300 MHz, CDCl3, ppm) δ: 1.10–1.43 (CH2CHCH2CH2), 1.55–2.26 (CH 2 CHCH 2 CH 2 ), 3.48–3.83 (OCH 2 CH), 5.55–5.81 (CH2CH=CHCH2), 7.18–7.86 (SiC 6 H 5 ).
13C NMR (300 MHz, CDCl3, ppm) δ: 24.8 (CH2CHCH2CH 2 ), 25.4 (CH2CHCH 2 CH2), 28.2 (CH 2 CHCH2CH2), 36.2 (CH2CHCH2CH2), 67.8 (OCH 2 CH), 126.2 (CHCH2CH=), 127.9 (=CHCH2CH2), 127.1, 130.4 and 134.9 (SiC 6 H 5 ).
29Si NMR (300 MHz, CDCl3, ppm) δ: −58.1.
Synthesis of tri(3,4-epoxycyclohexylmethyloxy) phenyl silane (TEMPS)
mCPBA (34.5 g, 0.20 mol) was dissolved in 300 mL of dichloromethane in a three-necked flask. TCMPS (21.9 g, 0.050 mol) dissolved in 100 mL of dichloromethane was then added dropwise into above solution at 0 °C using an ice bath, and reacted at room temperature for 24 h. After precipitation and filtration, the suspension was washed by 0.1 M aqueous Na2S2O3, saturated aqueous NaHCO3, and then distilled water. The obtained organic layer was dried by anhydrous Na2SO4. After filtration and evaporation to remove Na2SO4 and dichloromethane, the transparent liquid obtained was further purified by flash chromatography (hexane/ethyl acetate = 5:2), obtaining the final product in a yield of 74%, named TEMPS.
1H NMR (300 MHz, CDCl3, ppm) δ: 0.82–2.27 (CH 2 CHCH 2 CH 2 ), 3.05–3.26 (CH2CHCHCH2), 3.48–3.63 (OCH 2 CH), 7.34–7.64 (SiC 6 H 5 ).
13C NMR (300 MHz, CDCl3, ppm) δ: 20.5–34.7 (CH 2 CHCH 2 CH 2 ), 51.2 (CHCH2CH), 52.2 (CHCH2CH2), 66.9 (OCH 2 CH), 127.5, 130.1 and 134.2 (SiC 6 H 5 ).
29Si NMR (300 MHz, CDCl3, ppm) δ: −58.4.
UV cationic curing
The mixtures of TEMPS with DGEBA in different ratios (Table 1) were stirred until the homogenous blends formed. TEMPS and the mixtures in the presence of 4 wt% Irgacure 250 exposed to a medium pressure mercury lamp (2 kW, Fusion UV systems, USA) for 2 min and then thermally cured at 120 °C for 150 min to obtain the cured films.
Measurements
The Fourier transfer infrared (FTIR) spectra were recorded using a Nicolet MAGNA-IR 750 spectrometer. The liquid samples were filmed on the surface of NaCl crystal.
The 1H NMR, 13C NMR, and 29Si NMR spectra were recorded with an AVANCE 300 Bruker spectrometer using tetramethylsilane as an internal reference and CDCl3 as a solvent.
The thermogravimetric analysis (TG) was carried out with the sample of 3–5 mg on a Shimadzu TG-50 instrument using a heating rate of 10 °C min−1 in nitrogen or air atmosphere.
The limiting oxygen index (LOI) values were measured using a ZRY-type instrument (made in Jiangning, China) with the sheet of 120 × 6.5 × 3 mm3 according to ASTM D635-77.
The tensile storage modulus (E′) and tensile loss factors (tan δ) were measured using a dynamic mechanical thermal analyzer (Diamond DMA, PE Co., USA) at a frequency of 2 Hz and a heating rate of 5 °C min−1 in the range from −50 to 200 °C with the sheet of 25 × 5 × 1 mm3.
The mechanical properties were measured with an Instron Universal tester (model 1185, Japan) at 25 °C with a crosshead speed of 25 mm min−1. The dumb-bell-shaped specimens were prepared according to ASTM D412-87. Five samples were analyzed to determine an average value in order to obtain the reproducible result.
Results and discussion
Synthesis and characterization
In this study, the silicon-containing cycloaliphatic epoxide resin (TEMPS) was prepared in three steps, as presented Fig. 1, including the synthesis of unsaturated alcohol by the reduction of 3-cyclohexene-1-carboxaldehyde, the Lewis acid-catalyzed transesterification of trimethoxyphenyl silane with unsaturated alcohol, and then the epoxidation of double bond. In the titanium tetraisopropoxide-catalyzed transesterification, an excess of CM was used and the generated methanol was removed under reduced pressure during the reaction in order to enhance the reaction rate and achieve a higher yield. mCPBA was used to epoxidize the unsaturated intermediate, TCMPS, which gives almost complete conversion of double bond.
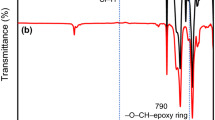
The obtained TCMPS and TEMPS were characterized by FTIR, 1H NMR, 13C NMR, and 29Si NMR spectroscopy. The FTIR spectra in Fig. 2 show the strong absorptions around 1068 and 1130 cm−1, which correspond to the Si–O–C and Si–C6H5 stretching, respectively. In the TCMPS spectrum, the absorption peaks around 1653 and 3021 cm−1 can be attributed to the vibration of alkene C=C and =C–H bonds in the cyclohexyl ring. In the TEMPS spectrum, the disappearance of peaks for alkene C=C and =C–H groups and the appearance of absorption peak at 808 cm−1 for epoxy group both confirm the expected structures of TCMPS and TEMPS.
Figures 3 and 4 show the 1H and 13C NMR spectra with their signal assignments. The chemical shifts of 1H NMR signals at 5.48–5.89 ppm and 13C NMR signals at 126.2 and 127.9 ppm indicate the presence of alkene in TCMPS, which are not present in TEMPS. After the epoxidation, new signals of the formed epoxide ring are observed at 3.03–3.26 ppm in the 1H NMR spectrum and at 51.2 and 52.2 ppm in the 13C NMR spectrum. Moreover, the integrated values of these peaks in the 1H NMR spectra match well to the numbers of hydrogen atoms in the TCMPS and TEMPS structures. It can be also observed that the number of peak in the 13C NMR spectrum for TEMPS is more than the number of carbon, which may be interpreted that different isomers of the epoxycyclohexyl rings are present in TEMPS. The details will be studied in the future. The further confirmation for the molecular structures was made by means of the 29Si NMR spectra. The single absorption bands with chemical shifts for the silane structure are shown in Fig. 5, indicating the expected structures both for TCMPS and TEMPS, as well their high purity.
Thermal degradation behavior
Thermogravimetric analysis (TG) is one of the commonly used techniques for rapid evaluation in comparing and ranking the thermal stability of various polymers. To examine the effect of silicon content on the thermal stability and decomposition behavior, TG data under nitrogen and air atmospheres were determined and analyzed. The resin compositions and the silicon contents were listed in Table 1.
Figures 6 and 7 show the mass loss thermograms (TG) and the differential mass loss (DTG) curves of UV-cured samples from room temperature to 850 °C recorded in nitrogen atmosphere, respectively. The specific degradation temperatures and the final char yields at 850 °C are listed in Table 2. The onset decomposition temperature, T 5%, of UV-cured film decreases along with the addition of TEMPS, from 280 °C for pure EP828 to 250 °C for TEMPS80 sample. From the DTG curves, it can be observed that all the UV-cured films mainly give a one-stage mass loss behavior. The sample with higher TEMPS content began to degrade at a lower temperature and show a broader mass loss temperature range. The temperatures at the maximum mass loss rate (T max1) decreases from 407 °C for the silicon-free resin to 373 °C for the resin containing 4.61% silicon. So the thermal stability decreases as the silicon content increases at a lower temperature. This can be ascribed to the presence of decomposable Si–O groups at lower temperature in the resins, as reported elsewhere [9]. However, the final char yield at 850 °C is significantly enhanced from 14 for EP828 to 25 for TEMPS80, as listed in Table 2. It can be explained by the fact that the decomposition of Si–O group at lower temperature resulted in the formation of silicone-containing group which will participate in the crosslinked carbonization and effectively retard the decomposition at higher temperature.
The TG and the DTG curves of the UV-cured samples recorded in air atmosphere are also investigated, as shown in Figs. 8 and 9. The onset decomposition temperatures, T 5%, decrease along with the increase of TEMPS content, showing a similar trend to the one mentioned above. The difference in degradation behavior of epoxy resins containing different contents of silicon can be observed from their DTG curves. The degradation of all the cationically UV-cured samples exhibits a two-step process based on the number of peaks in the DTG curves. For the first stage of decomposition, the temperature of the maximum mass loss rate, T max1, decreases slightly with the increase of TEMPS content, from 346 °C for EP828 to 334 °C for TEMPS. Moreover, the T max1 of TEMPS occurs at 26 °C lower than that in nitrogen atmosphere, which further indicates that the oxidation in breaking off the Si–O group results in the decomposition of TEMPS at a lower temperature. However, the Si–O unit was reported to absorb more thermal energy and its vibration can dissipate the thermal decomposition energy [18]. The decomposition of Si–O groups generates a silicon-containing residue, which slows down the decomposition in the second stage. Therefore, the second stage of mass loss temperature increases with the addition of TEMPS content. The temperature of the maximum mass loss rate, T max2, for TEMPS is observed at 590 °C, compared with the 514 °C for EP828. It can also be seen that the mass loss of the silicon-containing resins at high temperature region is less than that of cured EP828. The final char yield at 850 °C in air atmosphere also increases with increasing silicon content.
To sum up, the T 5% and T max1 both decrease with increasing TEMPS content either in air or nitrogen atmosphere due to the decomposition of Si–O unit. In the second process of decomposition, the T max2 increases with increasing TEMPS content because the silicon-containing group participates in the crosslinked carbonization and effectively retards the flame formation at higher temperature. The observed higher char yield for TEMPS further indicates that the mechanism of improved fire performance via silicon modification indeed plays an important role in flame retardation.
Flame retardance
The flame-retardant properties of UV-cured epoxy resins were further evaluated by measuring the LOI values. The LOI, as a qualitative method to rank the flammability of a material, is the minimum fraction of oxygen in an oxygen/nitrogen mixture that will just support the combustion. The LOI values of UV-cured TEMPS, EP828, and their blends are listed in Table 1. It can be observed that the LOI value increases with increasing silicon content from 22 for EP828 to 30 for TEMPS80. It is concluded that the addition of TEMPS can efficiently enhance the flame retardancy of EP828. The silicon-containing compounds are a family of condensed-phase flame retardants [9]. Their flame-retardant performance arise partly from the dilution function to more combustible organic gases, and partly from the barrier effect by the silicaceous residues formed in an advancing flame. While heating, the low surface energy of silicon migrates to the surface of coated film, following by the formation of a protective layer with high heat resistance. The high-performance char acts as an insulator and mass transport barrier, which can cut off the heat and oxygen transfer, and thus effectively improve the flame retardance of UV-cured resin.
Dynamic mechanical thermal properties
The dynamic mechanical thermal analysis (DMTA) was utilized to investigate the dynamic mechanical behavior in order to further acquire the microstructure information of a UV-cured film. Figure 10 shows the variation in the storage modulus and the relaxation peaks of loss factors as a function of temperature beginning from the glassy state to the rubbery plateau of cured films. The crosslinking density of the highly crosslinked UV-cured films can be estimated with the formula [19]: v e = E′/3RT, where E′ is the storage modulus in the rubbery state, R is the ideal gas constant, and T is the absolute temperature at T g + 50 °C. From the Table 3, it can be seen that the crosslink density increases from 4.18 for pure EP828 to 10.28 mmol cm−3 for pure TEMPS. As far as the chemical structures of these networks are concerned, this behavior is to be expected since the relative concentration of epoxy group in TEMPS is 6.20 mmol g−1, higher than that in EP828. Moreover, TEMPS is a trifunctional cycloaliphatic epoxide, while EP828 has only two glycidyl ether type epoxy groups per molecule with lower reactivity.
The glass transition temperature (T g) can be determined as the α relaxation peak of loss factor. The softening point (T s) is detected as the extrapolated onset of the drop of storage modulus. According to the theory reported elsewhere [19], the meaning of the crosslinking density is the molar number of elastically effective network chains per cube centimeter. A higher v e value means that the chains are highly restricted, which makes the chain motion possible at higher temperature. However, both the T g and T s of UV-cured films decrease with increasing TEMPS content, as listed in Table 3. This can be explained by the incorporation of more Si–O and Si–C bonds, resulting in the reduction in the rigidity of polymeric network, although the crosslinking density increased with the addition of TEMPS.
The width of relaxation peak of the loss factor indicates the homogeneity of UV-cured network, which can be expressed by T g/T s values of the cured sample. A little change is observed in T g/T s ratio after the addition of TEMPS, and the values of all samples are higher than 0.89. This uniformity of the tan δ peak indicates the absence of any network heterogeneity, which implies the good miscibility between EP828 and TEMPS.
Mechanical properties
The tensile strength and elongation-at-break are also listed in Table 3. With increasing TEMPS content, the tensile strength decreases from 49.4 MPa for EP828 to 22.1 MPa for TEMPS80, while the elongation-at-break slightly increases from 3.3% for EP828 to 4.9% for TEMPS80. In other words, with increasing the TEMPS content, the rubbery property of the cured film is improved. This can be explained by the presence of Si–O and Si–C groups in TEMPS, resulting in the polymeric backbone more flexible.
Conclusions
A novel silicon-containing cycloaliphatic epoxide resin was successfully synthesized by a simple method and used as a reactive-type flame retardant in cationic UV-curable systems. The flame retardancy was efficiently improved with the addition of TEMPS into the commercial epoxide resin EP828. The onset decomposition temperature and the temperature of maximum mass loss rate in the first stage decrease with increasing TEMPS content either in air or in nitrogen atmosphere. In the second process of decomposition, the temperature of maximum mass loss rate increases with the increase of TEMPS content. The silicon-containing group decomposes at lower temperature and participates in the crosslinked carbonization at higher temperature. The char yields under nitrogen and air atmospheres increased along with the silicon content. The LOI value increased from 22 for the commercial epoxide resin EP828 to 30 for the blend with 80% TEMPS. Moreover, TEMPS had good miscibility with EP828. The addition of TEMPS led to a decrease in tensile strength and an increase in elongation-at-break.
References
Calbo LJ. Handbook of coatings additives. New York: Marcel Dekker; 1986.
May CA, editor. Epoxy resins chemistry and technology. New York: Marcel Dekker, 1988.
Lin SC, Pearce PM. High-performance thermosets. New York: Hanser; 1994.
Sen AK, Mukheriee B, Bhattacharya AS, Sanghi LK, De PP, Bhowmick K. Preparation and characterization of low-halogen and nonhalogen fire-resistant low-smoke (frls) cable sheathing compound from blends of functionalized polyolefins and PVC. J Appl Polym Sci. 1991;43:1673–84.
Hsiue GH, Wang WJ, Chang FC. Synthesis, characterization, thermal and flame-retardant properties of silicon-based epoxy resins. J Appl Polym Sci. 1999;73:1231–8.
Hsiue GH, Liu YL, Tsiao J. Phosphorus-containing epoxy resins for flame retardancy V: synergistic effect of phosphorus–silicon on flame retardancy. J Appl Polym Sci. 2000;78:1–7.
Wang WJ, Perng LH, Hsiue GH, Chang FC. Characterization and properties of new silicone-containing epoxy resin. Polymer. 2000;41:6113–22.
Abad MJ, Barral L, Fasce DP, Williams RJJ. Epoxy networks containing large mass fractions of a monofunctional polyhedral oligomeric silsesquioxane (POSS). Macromolecules. 2003;36:3128–35.
Mecado LA, Reina JA, Galià M. Flame retardant epoxy resins based on diglycidyloxymethylphenylsilane. J Polym Sci A: Polym Chem. 2006;44:5580–7.
Mercado LA, Galià M, Reina JA. Silicon-containing flame retardant epoxy resins: synthesis, characterization and properties. Polym Degrad Stab. 2006;91:2588–94.
Spontón M, Mercado LA, Ronda JC, Galià M, Cádiz V. Preparation, thermal properties and flame retardancy of phosphorus-and silicon-containing epoxy resins. Polym Degrad Stab. 2008;93:2025–31.
Hsiue GH, Wei HF, Shiiao SJ, Kuo WJ, Sha YA. Chemical modification of dicyclopentadiene-based epoxy resins to improve compatibility and thermal properties. Polym Degrad Stab. 2001;73:309–18.
Decker C. Photoinitiated crosslinking polymerisation. Prog Polym Sci. 1996;21:593–650.
Fieberg A, Reis O. UV curable electrodeposition systems. Prog Org Coat. 2002;45:239–47.
Wu S, Sears MT, Soucek MD, Simonsick WJ. Synthesis of reactive diluents for cationic cycloaliphatic epoxide UV coatings. Polymer. 1999;40:5675–86.
Decker C, Viet TNT, Decker D, Weber-Koehl E. UV-radiation curing of acrylate/epoxide systems. Polymer. 2001;42:5531–41.
Wang HL, Liu JH, Xu SP, Shi WF. Preparation and film properties of tri(3,4-epoxycyclohexylmethyl) phosphate based cationically UV curing coatings. Prog Org Coat. 2009;65:263–8.
Kanai H, Sullivan V, Auerback A. Impact modification of engineering thermoplastics. J Appl Polym Sci. 1994;53:527–41.
Hill LW. Structure/property relationships of thermoset coatings. J Coat Technol. 1992;64:29–42.
Acknowledgements
The authors gratefully acknowledge the financial support of the National Natural Science Foundation of China (No. 50633010).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cheng, Xe., Shi, W. Synthesis and thermal properties of silicon-containing epoxy resin used for UV-curable flame-retardant coatings. J Therm Anal Calorim 103, 303–310 (2011). https://doi.org/10.1007/s10973-010-1053-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-010-1053-9