Abstract
In the present contribution, a procedure to estimate parameters using non-isothermal data was applied. The estimation procedure is based on the use of an energy balance in DSC furnace. The approach found all kinetic parameters of autocatalytic model (E 1, E 2, A 1, A 2, m, n) besides the ultimate reaction heat and their confidence regions by using deterministic and heuristic algorithms. The application of this approach to isothermal data was done in a previous work and similar results were obtained. The results show that the use of an energy balance is a good methodology to estimate cure kinetic parameters of non-isothermal experiments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A cure kinetic study of polymeric resin can be carried out in a calorimeter analysis, operating by two models: isothermal and non-isothermal. A way to estimate the kinetic parameters using any experimental data is the use of an objective function to be minimized. The algorithms frequently used to find the minimum are deterministic, but the application of the heuristic algorithms to estimate cure kinetic parameters have become a good way. Among these algorithms, the Particle Swarm Optimization (PSO) [1] has been extensively applied in parameter estimation. One important detail of these algorithms is that the knowledge of the objective function gradients and the initial parameter guesses are not necessary. Although a higher number of the objective function evaluation is necessary, this characteristic can be used to determine the parameter confidence limits.
Many authors have studied the kinetic parameter estimation of polymeric resin using both isothermal and non-isothermal data coming from DSC [2–7]. Kissinger [8], Borchardt and Daniels [9], and Ozawa [10] methods are the main ones used to estimate kinetic parameter by using non-isothermal data found in the literature [11–17]. However, Kissinger and Ozawa methods are generally applied to determine only the activation energy from experimental data.
The estimated activation energy and pre-exponential factor using isothermal and non-isothermal experimental data have values in agreement in the literature [18, 19], however, some papers have found differences among the kinetic parameters values using different analysis modes [11, 20–23]. The use of the energy balance in kinetic parameter estimation of epoxy resins was studied in isothermal case [24]. Thus, in this contribution, this procedure was applied to non-isothermal data and the PSO was applied to build the confidence regions of all estimated parameters.
Theoretical
Kinetic models
The main models used to express the cure kinetic for a polymeric resin shows the form:
where dα/dt is the cure kinetic rate, α is the curing degree of the resin, t is the curing time, and K 1 is the temperature-dependent rate constant given by Arrhenius equation:
where E 1 is the curing activation energy, T is the absolute temperature, A 1 is the pre-exponential factor, and R is the universal gas constant.
The cure kinetic expression for epoxy resins is based on the autocatalytic model [25], Eq. 3:
where m and n represent the catalytic and autocatalytic natures of the reaction, respectively, and K 2 follows the Arrhenius equation.
The ultimate reaction heat can be calculated through the area under the curve of the released reaction heat rate; but in this paper, this constant was estimated along with the kinetic parameters (E 1, E 2, A 1, A 2, m, n). The measured value of ultimate reaction heat is found by an integration method (commonly through the area under the experimental heat flow). This method can insert error in the parameter value because the area depends on chosen points, on the integration method, etc. For degree of cure, the analysis is similar because it can be found by the same way. Thus, it is important to avoid intermediate calculus using experimental data, minimizing so error propagation. For that, in the present paper, the adjustment is achieved using experimental data coming from DSC without any manipulation. The degree of cure and the ultimate reaction heat are calculated during the estimation process through the mass and energy balances in DSC.
Mathematical modeling
The kinetic model proposed by Kamal and Sourour [25], Eq. 4, was adopted herein.
where
Because of the correlation among the parameters, it was inserted in the mathematical system a dimensionless variables. This transformation and an appropriated reference temperature lead the estimation with lower correlation [26]. In terms of dimensionless variables, Eq. 4 is expressed by Eq. 5:
\( \varsigma = {\frac{t}{\tau }} \), \( \sigma_{i} = {\frac{{E_{i} }}{{RT_{0} }}} \), \( \Theta = \left( {{\frac{{T - T_{0} }}{{T_{0} }}}} \right) \), \( \xi_{i} = \ln (\tau A_{i} ) - \sigma_{i} \), where T 0 is a reference temperature and τ is a reference time for integration.
Six parameters must be estimated to define the cure kinetics. The dimensionless parameters are related to dimension parameters by the following expressions:
Particle swarm optimization
The PSO is an optimization algorithm based on the social behavior of an animal collection. This algorithm uses information about the best solution found by each particle and all particles. Kennedy and Eberhart [27] proposed the first approach to this technique which can be mathematically described by Eqs. 6 and 7.
where v is the particle pseudovelocity vector; x is the particle position vector; \( x_{\text{local}}^{k} \) and \( x_{\text{global}}^{k} \) are the best solutions found by each particle and by all particles, respectively; r 1 and r 2 are two random numbers in the range [0,1]; c 1 and c 2 are the search parameters and superscript k denotes the iteration index. The parameter w is calculated by \( w = w_{i} + \left( {w_{f} - w_{i} } \right){\frac{i}{{n_{\text{iter}} }}} \), with w f and w i as the weight parameters, which control the method convergence, and n iter is the number of iterations. More details about the application of this algorithm can be found elsewhere [24].
Experimental data
The system used was an epoxy resin, diglicidyl ether of bisphenol-A (DGEBA), with a commercial name of DER 331, and a hardener, triethylenetetramine (TETA), both from Dow Chemical Company. The epoxy equivalent weight was 183 g/eq DGEBA and TETA were used as received, without further purification. A ratio of 13% (stoichiometric ratio) was used. DGEBA was vigorously mixed with the appropriate amount of TETA for about 1 min, at room temperature.
Non-isothermal experiments were run in a Perkin-Elmer, DSC model Diamond. The heating rates, ϕ, on the experiments were 5, 10, 15, and 20 K min−1. Approximately, 10 mg of the mixture were encapsulated in a standard aluminum sample pan. The instrument was calibrated using indium as a standard. The gas used was nitrogen at 20 mL min−1. More details can be found elsewhere [28].
Mathematical modeling
The energy balance of the sample inside the calorimeter was taken into consideration. A convective loss was also considered in the system. Thus, considering a sample inside the calorimeter, in the non-isothermal case, we can write an energy balance as
In non-isothermal case, considering c p and m e constant, the energy balance is:
where Q is heat rate leaving DSC, (−∆H) is the ultimate reaction heat of system, m e is the sample mass, c p is the sample specific heat, h is the heat transfer coefficient, A is the heat transfer area, T ∞ is the ambient temperature, and R α is a cure kinetic expression.
The last term of right side is related to convective loss in the process. In a dimensionless form, we can write Eq. 8 as
and
where η is the dimensionless reaction heat, η = (−ΔH)/c p T 0, q is the dimensionless heat rate, q = Qτ/m e c p T 0, β is the dimensionless heating rate, β = ϕτ/T 0, γ is the dimensionless heat transfer coefficient, γ = hAτ/(m e c p ), τ is the reference time, ς = t/τ, Θ is the dimensionless temperature, Θ = (T − T 0)/T 0, and T 0 is the reference temperature.
Results and discussion
The system composed by Eqs. 9, 10, and 11 is an index 1 differential–algebraic equation (DAE) system and it can be solved by an appropriated numerical package. In the present paper, the code DASSL [29] was applied to find the variables used in the estimation process.
In order to estimate the kinetic parameter (E 1, E 2, A 1, A 2, m, n) and the ultimate reaction heat, the ESTIMA [30], which is a computational package composed by heuristic algorithm (swarm) and a deterministic algorithm (Gauss–Newton), was applied to find the minimum of objective function described by Eq. 12.
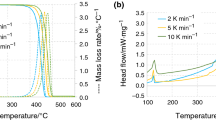
The value of ultimate reaction heat was estimated along with the kinetic parameters in the present paper as described by Pagano et al. [24]. This procedure reduces estimation errors as the objective function is directly associated with the released reaction heat rate. Table 1 has the dimensionless parameters and Fig. 1 shows the comparison between experimental and estimated values of heat flow obtained by applying this methodology. A good prediction was achieved for all experiments data, although the results coming from lower heating rate (5 K min−1) showed a small difference in the beginning of analysis.
Owing to importance of knowledge of the estimated parameter certainty, the confidence regions of all parameters were determined. It was considered a confidence level of 95% and the square error function described by Eq. 12. The parameter set that composes the confidence region was chosen according to Eq. 13, as suggested by Schwaab et al. [31]
where p* is a vector that contains the best parameters, p is a vector with parameters that will be tested, n p is the number of parameters, n is the total number of data and \( F_{{n_{p} ,n - n_{p} }}^{1 - \delta } \) is the F-distribution with n p and n − n p degrees of freedom and a confidence level of 1 − δ. More details of this procedure can be found elsewhere [31].
The application of this approach to isothermal data was done in a previous work and similar results were obtained [24]. The confidence regions for each estimated parameter in the non-isothermal case can be seen in Fig. 2a–d.
One of hypothesis used in parameter estimation is the absence of correlation among the parameters to be estimated. If that hypothesis is true, the confidence region must have a symmetrical and elliptical shape. Considering these observations, some points must be highlighted about the results showed in Fig. 2. First, the confidence regions show an angle to the main axes because the estimated parameters are correlated. Other point is related to the region shape; we can see that the elliptical region is a bad approximation for these confidence regions. Lastly, the estimated parameters, the crosses in Fig. 2a–d, are not in the geometric center of the regions as expected in linear models. Thus, the use of the energy balance claims to be a good methodology to estimate cure kinetic parameters of non-isothermal experiments.
Conclusions
A differential–algebraic approach was applied to estimate the kinetic parameters of epoxy resin. The released reaction heating rates measured in DSC were directly used as variable to be adjusted. This approach was very suitable to parameter estimation and an excellent agreement between experimental and predicted data was achieved. The application of the PSO algorithm to minimize objective function was especially suitable for estimation purposes and, at the same time, allowed the construction of correspondent confidence regions for the estimated parameters. The ultimate reaction heat, obtained by numerical integration of the reaction heating rate conducted isothermically in DSC experiments, lies outside the confidence region of the reaction heat estimated in the proposed method. This fact, along with the high quality of the parameter adjustments, indicates that the proposed methodology is more reliable. It must be pointed out that the use of dimensionless variables and an appropriate reference temperature increase their accuracy because this procedure results in a low parameter correlation.
References
Eberhart RC, Shi Y, Kennedy J. Swarm intelligence. 1st ed. San Francisco, CA: Morgan Kaufmann; 2001.
Wang CS, Kwag C. Cure kinetics of an epoxy-anhydride-imidazole resin system by isothermal DSC. Polym Polym Compos. 2006;14:445–54.
Sbirrazzuoli N, Girault Y, Elegant L. Simulations for evaluation of kinetic methods in differential scanning calorimetry. Part 3—peak maximum evolution methods and isoconversional methods. Thermochim Acta. 1997;293:25–37.
Kosar V, Gomzi Z. Cure modeling of polyester thermosets in the copper mold. Polym Plast Technol. 2004;43:1277–98.
Jubsilp C, Damrongsakkul S, Takeichi T, et al. Curing kinetics of arylamine-based polyfunctional benzoxazine resins by dynamic differential scanning calorimetry. Thermochim Acta. 2006;447:131–40.
Ramis X, Salla JM, Cadenato A, et al. Simulation of isothermal cure of a powder coating—non-isothermal DSC experiments. J Therm Anal Calorim. 2003;72:707–18.
Rosu D, Mititelu A, Cascaval CN. Cure kinetics of a liquid-crystalline epoxy resin studied by non-isothermal data. Polym Test. 2004;23:209–15.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29:1702–6.
Borchardt HJ, Daniels F. The application of differential thermal analysis to the study of reaction kinetics. J Am Chem Soc. 1957;79:41–6.
Ozawa T. A new method of analyzing thermogravimetric data. Bull Chem Soc Jpn. 1965;38:1881–6.
He Y. DSC and DEA studies of underfill curing kinetics. Thermochim Acta. 2001;367:101–6.
Atarsia A, Boukhili R. Relationship between isothermal and dynamic cure of thermosets via the isoconversion representation. Polym Eng Sci. 2000;40:607–20.
Rosu D, Cascaval CN, Mustata F, et al. Cure kinetics of epoxy resins studied by non-isothermal DSC data. Thermochim Acta. 2002;383:119–27.
Alonso MV, Oliet A, Perez JM, et al. Determination of curing kinetic parameters of lignin-phenol-formaldehyde resol resins by several dynamic differential scanning calorimetry methods. Thermochim Acta. 2004;419:161–7.
Perez JM, Rodriguez F, Alonso MV, et al. Curing kinetics of lignin-novolac phenolic resins using non-isothermal methods. J Therm Anal Calorim. 2009;97:979–85.
Kumari K, Raina KK, Kundu PP. DSC studies on the curing kinetics of chitosan-alanine using glutaraldehyde as crosslinker. J Therm Anal Calorim. 2009;98:469–76.
Doca N, Vlase G, Vlase T, et al. TG, EGA and kinetic study by non-isothermal decomposition of a polyaniline with different dispersion degree. J Therm Anal Calorim. 2009;97:479–84.
Zvetkov ZL. Comparative DSC kinetics of the reaction of DGEBA with aromatic diamines. I. Non-isothermal kinetic study of the reaction of DGEBA with m-phenylene diamine. Polymer. 2001;42:6687–97.
Zvetkov ZL. Comparative DSC kinetics of the reaction of DGEBA with aromatic diamines. II. Isothermal kinetic study of the reaction of DGEBA with m-phenylene diamine. Polymer. 2002;43:1069–80.
Barral L, Cano J, Lopez J, et al. Blends of an epoxy/cycloaliphatic amine resin with poly(ether imide). Polymer. 2000;41:2657–66.
Lopez J, Lopez-Bueno I, Nogueira P, et al. Effect of poly(styrene-co-acrylonitrile) on the curing of an epoxy/amine resin. Polymer. 2001;42:1669–77.
Rosu D, Mustata F, Cascaval CN. Investigation of the curing reactions of some multifunctional epoxy resins using differential scanning calorimetry. Thermochim Acta. 2001;370:105–10.
Sbirrazzuoli N, Mititelu-Mija A, Vincent L, et al. Isoconversional kinetic analysis of stoichiometric and off-stoichiometric epoxy-amine cures. Thermochim Acta. 2006;447:167–77.
Pagano RL, Calado VMA, Tavares FW, et al. Cure kinetic parameter estimation of thermosetting resins with isothermal data by using particle swarm optimization. Eur Polym J. 2008;44:2678–86.
Kamal MR, Sourour S. Kinetics and thermal characterization of thermoset cure. Polym Eng Sci. 1973;13:59–64.
Schwaab M, Pinto JC. Optimum reference temperature for reparameterization of the Arrhenius equation. Part 1: problems involving one kinetic constant. Chem Eng Sci. 2007;62:2750–64.
Kennedy J, Eberhart RC. Particle swarm optimization. Proc IEEE Int Conf Neural Netw. 1995;4:1942–8.
Costa CC, Pagano RL, Calado VMA, et al. Viscoelastic response and cure kinetic of a bisphenol a-derived epoxy. J Polym Mater. 2007;24:20–36.
Brenan KE, Campbell SL, Petzold LR. Numerical solution of initial-value problems in differential-algebraic equations. 2nd ed. Philadelphia: SIAM; 1989.
Pinto JC, Lobao MW, Monteiro JL. Sequential experimental-design for parameter-estimation—analysis of relative deviations. Chem Eng Sci. 1991;46:3129–38.
Schwaab M, Biscaia EC, Monteiro JL, et al. Nonlinear parameter estimation through particle swarm optimization. Chem Eng Sci. 2008;63:1542–52.
Acknowledgements
Authors would like to thank CAPES, CTPETRO/FINEP (# 0104081500), and CNPq for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pagano, R.L., Calado, V.M.A., Tavares, F.W. et al. Parameter estimation of kinetic cure using DSC non-isothermal data. J Therm Anal Calorim 103, 495–499 (2011). https://doi.org/10.1007/s10973-010-0984-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-010-0984-5