Abstract
Pooling lauroyl peroxide (LPO) with nitric acid, we used differential scanning calorimetry (DSC) to assess the thermokinetic parameters, such as exothermic onset temperature (T 0), heat of decomposition (ΔH d), frequency factor (A), and the other safety parameters. When LPO was contaminated with nitric acid (HNO3), we found the exploder 1-nitrododecane. Obvious products were sensitive and hazardous chemicals. Concentration reaching 1–12 N HNO3 emitted a large amount of heat. This study combined with curve-fitting method to elucidate its unsafe characteristics and thermally sensitive structure to help prevent runaway reactions, fires and explosions in the process environment. According to the findings and the concept of inherently safer design, LPO runaway reactions could be adequately prevented in the relevant plants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Many accidents are caused by thermal runaway reactions [1, 2]. One of the most famous was a disastrous release of a large volume of methyl isocyanate gas from a Union Carbide plant in Bhopal, India, in December 1984 [3]. Thermal runaway occurs when the reaction rate increases due to an increase in temperature, causing a follow up increase in pressure and hence a further increase in the reaction rate. Thermal runaway is also a concern in hydrocracking, an oil refining process [4]. In the chemical industry, many production processes involve exothermic reactions. Thermal runaway may result from exothermic side reactions that begin at higher temperatures, followed by an initial accidental overheating of the reaction mixture. Understanding of runaway reactions is necessary to predict and govern hazards. A detailed kinetic modeling approach is proposed to simulate runaway reactions under process conditions.
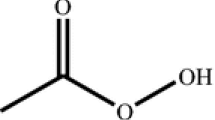
Organic peroxides are characterized by the presence of a sensitive oxygen–oxygen bond. Especially, contact with acids, alkalis, heavy metals, reducing agent, and any rust should be avoided. Shock, friction, fire or other sources of ignition should be also avoided during a manufacturing process [5–8]. Many thermal explosion incidents caused by organic peroxide have occurred in Taiwan [9]. In practice lauroyl peroxide (LPO) [4, 10] is one of the typical organic peroxides. In our studies, runaway reactions of LPO with HNO3 involve a highly exothermic peak that could induce a thermal accident and damage the process equipment and the surroundings. It is very important to make clear the safety parameters and reaction situation for LPO mixing with contaminant.
Kotoyori [11] establishes a self-accelerating decomposition theoretical model, the self-accelerating decomposition temperature (SADT) of LPO as approximately 48 °C. Due to hazardous consequences of decomposition of LPO with acids and its extensive use in the industry, we have made an effort to understand the runaway reaction phenomenon by evaluating the kinetic parameters.
Safety parameters must be taken into account for evaluating the degree of hazard based on the thermokinetic parameters, such as exothermic onset temperature (T 0), activation energy (E a), frequency factor (A), etc. The decomposition of LPO with HNO3 produces a strong exothermic peak belonging to an nth order reaction. This study has proposed a method to generalize the concept of runaway reaction regarding LPO with HNO3 by employing differential scanning calorimetry (DSC) as a quantitative measure of reactivity, allowing one to take proper account of kinetic complexity. On the basis of the curve-fitting method and experimental results, we found that a quickly appearing exothermic peak indicated its dangerous features. Therefore, to prevent accidents from occurring, thermal hazard information should be provided to plant personnel in order to alleviate the degree of hazard, and also to the process engineers to design a safer control system, such as the cooling system, alarm system, interlock system, etc. [12].
Application of safety parameters
Safety is the most important element during storage or transport of chemicals, so safety parameters must be precisely evaluated. Then one can decide whether a cooling system should be established or not. These chemicals are stored in a container that could be exposed to a thermal source, self-decomposition, or unexpected mixing with contaminants to prompt runaway reactions, such as fire or explosion. In the absence of proper controls, a serious accident could thus be triggered during the upset situations.
According to the methodologies proposed by Townsend and Tou [13] and Fisher and Goetz [14], important safety parameters, such as time to maximum rate (TMR), temperature of no return (T NR), and SADT, could be employed to evaluate the degree of hazard to reduce an unacceptable contingency. The results obtained are suitable for the study of the phenomenon of runaway reactions involving pure substances or mixtures. These safety parameters are described below.
TMR
LPO with HNO3 has a highly exothermic peak during the decomposition reaction; therefore, TMR is an adequate indicator to study the variation between time and temperature in four different heating rates (1, 2, 4, and 10 °C min−1).
The calculation method by Townsend and Tou is shown in Eqs. 1 and 2 [10]:
Thermokinetic parameters via experimental results belong to nth order reaction and only have one exothermic peak for appropriating this method.
T NR
During an upset situation a suitable and deliverable emergency response must be adopted. T NR is an important index in common use, which could be calculated via the relationship between the rates of heat generation and heat removal [15]. It could be applied to design a cooling system and to inform fire fighters about the remaining time to conduct a rescue operation [16], because T NR increases with the temperature of runaway reaction. T NR can be computed by Eqs. 3 and 4 given below:
SADT
SADT is extensively employed to estimate the decomposition reaction of thermally unstable materials, such as the O–O bond of organic peroxide, which is defined as the minimum ambient air temperature at which a self-reactive substance of specified stability (contaminant level, inhibitor concentration, vessel volume filled ratio, etc.) undergoes an exothermic reaction in a specified commercial package in a period of 7 days or less. A self-reactive substance must be subject to temperature control, inhibitor, improving the materials, and volume for a container during transportation if its SADT is less than or equal to 50 °C [17, 18]. Equation 5 could be used to calculate this safety parameter:
Experimental setup
Sample preparation
Ninety five mass% LPO was purchased directly from the Fluka Co., and then stored in a refrigerator at 4 °C. Four types of inorganic acids were selected to combine with LPO for studying its incompatible reactions—1, 2, 6, and 12 N HNO3—as the contaminants of interest. The original concentration of HNO3 was 65 mass%, and then distilled water was used as the thinner for allocating the above-mentioned concentrations. HNO3 was obtained from Hayashi Pure Chemical Ind., Co., Ltd. in Japan. The mixture reactions were stirred at room temperature and then put into the device for testing.
DSC
Temperature-programmed screening experiments were performed (Mettler TA8000 system) and coupled with a measuring cell that could withstand relatively high pressure to approximate 100 bar (DSC 821e). STARe software was used to obtain thermal curves. DSC has been calibrated with regard to both heat flux and temperature. For better thermal equilibrium, the scanning rate chosen for the temperature-programmed ramp was 4 °C min−1. The range of temperature rise was chosen from 30 to 300 °C for each condition of experiments. This apparatus could properly acquire the heat flow, and then via experimental data to calculate the T 0, T max, and ΔH d in the interior function of DSC [19]. Processing of experimental data and kinetic evaluation were implemented by applying TDPro and Fork software developed by CISP Ltd. [20].
Results
Different concentrations of HNO3 were used as the contaminants, mixed with 95 mass% LPO for DSC. The experimental results are displayed in Tables 1 and 2 and Figs. 1, 2, 3. In the investigation, mixing with contaminant acids showed a strong phenomenon. 12 N HNO3 caused a high degree of hazard during the experiments. In the thermal decomposition process we newly found the detonation product 1-nitrododecane. In the 12 N nitric acid contamination even more thermal energy was emitted.
Discussion
Curve-fitting analyses based upon experimental data
Thermokinetic parameters can be established by selecting an appropriate kinetic model to identify its basic characteristics as given in Eqs. 6–10. Curve-fitting reflected similar data as the experimental data.
The model for decomposition of LPO 95 mass% has only one stage:
The following mathematical model describes an nth order reaction:
where α is the degree of conversion, r and \( Q_{\text{i}}^{\infty } \) denote reaction rate and heat effect of the stage, respectively; dQ/dt is the overall heat generating rate; f denotes kinetic function for the stage that depends on the extent of conversion; k i(T) obeys the Arrhenius temperature dependency of the rate constant: k i(T) = A exp(−E a/RT); A and E a represent, respectively, the frequency factor and the activation energy of the stage; R is the gas constant (R = 8.314 J mol−1 K−1).
After the thermokinetic parameters were evaluated by curve-fitting from the above model, all the data indicated that the decomposition of LPO with acids was dangerous reaction, because in the temperature range of 30–300 °C, its activation energy (E a) was ca. 124–178 and 160–200 kJ mol−1 in the conditions of LPO mixed with 1, 2, 6, and 12 N HNO3 for the first and second peak, respectively. All the values of E a were less than 220 kJ mol−1. To precisely acquire the thermokinetic parameters, such as E a, frequency factor (A), reaction order (n), and heat of decomposition (ΔH d), it was important to have a good base line to integrate the exothermic peak for each of the thermal curves.
In thermal analysis for loss prevention, safety parameters are the important index for classifying the degree of hazard for LPO with HNO3. Hazardous characteristics of LPO and the effect of HNO3 can be determined by comparing TMR. Figure 3 displays the TMR of temperature calculated on the basis of the kinetic models. TMR is treated as a measure of probability of runaway development if an accident occurs. The temperature range was set at 20–100 °C; LPO mixed with HNO3 was most likely to trigger a runaway. When temperature was higher than 40 °C, TMR was approached in less than a day. Since LPO with HNO3 had two exothermic reactions, SADT and T NR could not be calculated by mathematical model directly. HNO3 must be well separated while one is dealing with LPO in the manufacturing process.
Conclusions
This study focused on LPO with specific inorganic acids, here HNO3, for testing the specific reactive hazard. The characteristic hazardous runaway reaction relationship was explored for LPO with four different concentrations by using experimental and curve-fitting methods. LPO with acids was unstable due to its thermally reactive structure and hence cannot be used without sound safety systems. According to the experimental results, LPO is very sensitive to inorganic acids, especially at high concentrations 12 N. The degree of hazard increases significantly if the temperature and contaminants are not well dictated. Therefore, for prevention of LPO accidents, the safety information discussed in this paper must be brought to the notice of all the relevant plants. It is important to separate storage as that can reduce personal injury and damage caused in case of fires, spills or leaks. LPO should be kept away from incompatible materials such as strong acids and bases, other oxidizing materials, flammable or combustible liquids.
Abbreviations
- A :
-
Frequency factor (m3 mol−1 s−1)
- C p :
-
Heat capacity (J g−1 K−1)
- E a :
-
Activation energy (kJ mol−1)
- ΔH d :
-
Heat of decomposition (J g−1)
- m :
-
Total mass of reactant (g)
- n :
-
Reaction order (dimensionless)
- P :
-
Pressure (psig)
- P max :
-
Maximum pressure (psig)
- Q :
-
Total heat of decomposition (kJ kg−1)
- Q t :
-
Decomposition heat released at time t (kJ kg−1)
- \( Q_{\text{i}}^{\infty } \) :
-
Heat effect of the stage (dimensionless)
- R :
-
Gas constant (8.31415 J k−1 mol−1)
- r 1 :
-
Reaction rate of first stage (g s−1)
- r 2 :
-
Reaction rate of second stage (g s−1)
- SADT:
-
Self-accelerating decomposition temperature (°C)
- T :
-
Absolute temperature (°C)
- ΔT ad :
-
Adiabatic temperature rise (°C)
- T max :
-
Temperature at which the peak point occurs (°C)
- TMR:
-
Time to maximum rate (day)
- T NR :
-
Temperature of no return (°C)
- T 0 :
-
Exothermic onset temperature (°C)
- U :
-
Heat transfer coefficient (kJ min−1 m−2 K−1)
- α i :
-
Degree of conversion (dimensionless)
- β :
-
Heating rate (°C min−1)
References
Chen KY, Wu SH, Wang YW, Shu CM. Runaway reaction and thermal hazards simulation of cumene hydroperoxide by DSC. J Loss Prev Process Ind. 2008;21(1):101–9.
Ho TC, Duh YS, Chen JR. Case studies of incidents in runaway reactions and emergency relief. Process Saf Prog. 1998;17(4):259–62.
http://news.bbc.co.uk/onthisday/hi/dates/stories/december/3/newsid_2698000/2698709.stm.
Wei C, Saraf SR, Rogers WJ, Mannan MS. Thermal runaway reaction hazards and mechanisms of hydroxylamine with acid/base contaminants. Thermochim Acta. 2004;421(1–2):1–9.
Wu LK, Chen KY, Cheng SY, Lee BS, Shu CM. Thermal decomposition of hydrogen peroxide in the presence of sulfuric acid. J Therm Anal Calorim. 2008;93:115–20.
Hou HY, Duh YS, Lin WH, Shu CM. Reactive incompatibility of cumene hydroperoxide mixed with alkaline solutions. J Therm Anal Calorim. 2006;85:145–50.
Duh YS, Wu XH, Kao CS. Hazard ratings for organic peroxides. Process Saf Prog. 2008;27:89–99.
Safety and handling of organic peroxides: a guide, organic peroxide producers safety division. The Society of the Plastics Industry (SPI), Inc., Washington, DC, USA, 1999.
Amparo CM, Jose MHM, Carlos MF, Ernesto FSA. Lauroyl peroxide as thermal initiator of lauryl methacrylate monolithic columns for CEC. Electrophoresis. 2007;29:4399–406.
Reimers JL, Schork FJ. Lauroyl peroxide as a cosurfactant in miniemulsion polymerization. Ind Eng Chem Res. 1997;36(4):1085–7.
Kotoyori T. The self-accelerating decomposition temperature (SADT) of solids of the quasi-autocatalytic decomposition type. J Hazard Mater. 1999;64:1–19.
Tseng JM, Shu CM, Horng JJ, Kuan CM, Hsu HI. Planning an emergency response center in southern Taiwan Science Park. Process Saf Environ Prot. 2007;85(B2):125–32.
Townsend DI, Tou JC. Thermal hazard evaluation by an accelerating rate calorimeter. Thermochim Acta. 1980;37:1–30.
Fisher HG, Goetz DD. Determination of self-accelerating decomposition temperatures for self-reactive substances. J Loss Prev Process Ind. 1993;6:183–94.
Bowes PC. Self-heating: evaluating and controlling the hazards. New York: Elsevier Science Publishing Company, Inc; 1984.
Kossoy AA. Inherently safer and assessment of reaction hazards technology workshop. Yunlin, Taiwan, ROC; 2002.
Malow M, Wehrstedt KD. Prediction of the self-accelerating decomposition temperature (SADT) for liquid organic peroxides from differential scanning calorimetry (DSC) measurements. J Hazard Mater. 2005;120(11):21–4.
Fisher HG, Goetz DD. Determination of self-accelerating decomposition temperature using the accelerating rate calorimeter. J Loss Prev Process Ind. 1991;4:305–16.
STARe software with Solaris operating system, Operating instructions; Mettler Toledo: Sweden, 2004.
Kossoy AA, Koludarova E. Specific features of kinetics evaluation in calorimetric studies of runaway reactions. J Loss Prev Process Ind. 1995;8(4):229–35.
Acknowledgements
The authors are indebted to the donors of the National Science Council (NSC) in Taiwan under the contract No. NSC 96-2628-E-224-003-MY3 for financial support. The authors also gratefully acknowledge Dr. Arcady A. Kossoy of ChemInform Saint Petersburg (CISP), Ltd., St. Petersburg, Russia for providing technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
You, ML., Tseng, JM., Liu, MY. et al. Runaway reaction of lauroyl peroxide with nitric acid by DSC. J Therm Anal Calorim 102, 535–539 (2010). https://doi.org/10.1007/s10973-010-0934-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-010-0934-2