Abstract
The process of oxygen chemisorption on coal in the temperature range ≈150–300 °C was studied under different experimental conditions using TG-DSC apparatus. As changing experimental conditions, oxygen flow (20 or 200 cm3 min−1), material of crucible (α-Al2O3 or Pt–Rh alloy), and initial sample mass (2–13 mg) were examined with respect to reliability and reproducibility of the parameters derived from TA curves. As parameters quantifying coal oxidation, temperatures of minimal T min and maximal T max sample mass, mass changes (mass loss W H below T min and mass increase W O above T min), heat evolution during oxygen chemisorption Q O (related to the coal mass increase), and kinetic parameters (activation energy E and frequency factor A) were evaluated. Values of T max, E, and A were found to lie in very close intervals independently on experimental conditions (95% confidence intervals were T max = 270.2 ± 0.7 °C, E = 81 ± 3 kJ mol−1, log10 A = 5.9 ± 0.3 s−1). Thus, these parameters can be used as actual characteristics of oxygen chemisorption stage of coal oxidation irrespective on conditions of TA measurements. Opposite, parameter Q O was confirmed to depend clearly on initial sample mass. The dependence is different for crucible materials used; however, it tends to the same value (≈50 kJ g−1) with increasing sample mass. Further, precision of values W H, W O, and T min determined from TG was found to be poor. This fact complicates evaluation of the effect of experimental conditions. Finally, the effect of oxygen flow on all above parameters was found to be negligible. Its influence (if any) was hidden by common experimental errors.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Thermal analysis (TA) is a well-established experimental approach to study coal combustion process as a type of coal–oxygen interaction taking place at medium and high temperature levels (T > 300 °C) [1–3]. Simultaneously, attention is paid to TA investigation of coal oxidation within low temperature range (from ambient to cca 300 °C). Its history began in the middle of the twentieth century, when Oreshko, using thermogravimetry, identified several stages of coal aerial oxidation at temperatures to 200 °C [4]. From that time, thermal analysis (including thermogravimetry TG, differential thermal analysis DTA or differential scanning calorimetry DSC) became widely used approach and provides valuable information concerning both propensity of coal to self-heating and mechanistic pathway of spontaneous combustion process [5–7]. Especially, mass changes of coal matter during heating, onset temperatures, and kinetic parameters derived from TG measurements are often evaluated for this purpose (e.g., [8, 9]).
In spite of the long-term utilization of thermoanalytical methods for characterization of low-temperature interaction of coal with oxygen, the adequate attention to reliability and reproducibility of the parameters derived from the measurements has not been paid yet.
The aim of this study is to asses the effect of selected experimental conditions (sample mass, oxygen flow, and material of crucibles) on parameters derived from TG-DSC investigations of oxidation of coal at low-temperature range.
Experimental
Sample of typical, high volatile bituminous coal of Ostrava-Karviná Coal Mines District (Czech Republic) was used for the experiments. Table 1 summarizes basic characteristics of the coal. Fraction with particle size less than 0.2 mm was used for TG-DSC experiments.
Simultaneous TG-DSC measurements were performed on Netzsch STA 449 C instrument with constant heating rate of 5 K min−1 in the range 30–500 °C without standard in dynamic atmosphere (oxygen). The effect of gas flow was tested on two levels—20 and 200 cm3 min−1. Two types of crucibles were used—from α-Al2O3 and Pt–Rh alloy (both original DSC-TG crucibles provided by Netzsch). Weight calibration was performed before measurements. Temperature and sensitivity calibration of the apparatus was performed separately for both crucible materials using calibration sets provided by Netzsch. The influence of sample mass was studied in the range about 2–13 mg. In total, 26 measurements were performed for different combinations of gas flow, crucible material, and sample mass.
Results and discussion
Parameters derived from TG curve
Typical mass change of the coal sample heated under oxidative conditions is demonstrated in the Fig. 1.
From the Fig. 1, mass decrease up to 150 °C is obvious within the low-temperature range (from ambient to about 270 °C). The decrease can be explained by natural humidity loss. Simultaneously, however, other processes like evaporation of volatile matter, decomposition, and/or oxidation of carbonaceous matter to gaseous products (hydrocarbons, CO, CO2) can contribute to the decrease of coal mass. On the other hand, increase of coal mass (in the low-temperature range) can be exclusively explained by oxygen chemisorption accompanied with coal surface oxidation and formation of surface oxygen containing less or more stable species (ethers, carbonyls, carboxyls) [5].
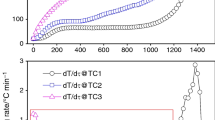
Parameters directly evaluated from TG and/or DSC experimental curves included temperatures of minimal T min and maximal T max sample mass, mass loss W H below T min, mass increase W O above T min (caused by chemisorbed oxygen) (Fig. 2). Heat evolution during oxygen chemisorption Q estimated by integration of DSC in the range T min–T max will be discussed below.
The first view on the thermogravimetric data collected for the same coal sample under different experimental conditions (Fig. 3) shows unexpected wide intervals of determined values of W H (0.9–7.5%), T min (110–190 °C), and partially of W O (2.2–4.4%). On the other hand, determined values of T max lie in narrow interval 265–275 °C (except of one value from measurement with very low initial sample mass).
This huge variability of W H, W O, and T min values is probably caused by extensive dependency of both overlapping processes controlling mentioned parameters on the “physical arrangement” of measured samples (size and shape of particles, geometry of the sample, packing,…) affecting the internal diffusion of oxygen and gaseous products through the sample and determining the surface available for reactions during dynamic heating. Natural heterogeneity of coal can also contribute to the parameters inconstancy, especially in the range of small mass of sample (to cca 5 mg).
These factors are often negligible for common reactions but in this special case of two slow, overlapping and opposite-directed reactions can probably highly influence the obtained results. This effect becomes less influential with increasing sample mass and measured values are less “noisy” especially for W H and W O.
Stability of parameter T max is understandable, because it indicates temperature of beginning of coal combustion—very fast process (in comparison with previous) which starts with degradation of “bulk” structure of coal, and therefore, it is not so dependent on internal diffusion and surface available for heterogeneous reaction.
Variability of parameters W H, W O, and T min practically disables evaluation of the effect of studied experimental conditions on their values determined from TG measurements. Thus, they are not suitable for TG evaluation of low-temperature oxidation of coal.
Parameter T max is independent on conditions used for measurement (in the range of studied conditions), and it can be recommended as actual characteristics of oxygen chemisorption stage of coal oxidation. For the studied coal, value T max = 270.2 ± 0.7 °C was ascertained (95% confidence interval with excluding the lowest value as outlier).
Kinetic parameters
Process of low-temperature coal oxidation involves a large number of individual reactions running at the same temperature range [5], and their kinetic parameters are not experimentally available (at present state of our knowledge). In addition, the situation is complicated by the fact that the coal–oxygen interaction leads to both gaseous and solid oxidation products, which both affect measured mass changes [10]. The only parameters, we are able to determine, describe the process as whole and therefore have to be understood as not “true” but “observed” or “effective.” Usability of them to predictions or mechanistic speculations is limited but they characterize dynamics of the whole oxidation process and can be used as characteristics of coal oxidation behavior (e.g., activation energy as the measure of the dependence of oxidation rate on temperature).
Within the study, kinetic parameters (activation energy E, frequency factor A, reaction order n) were determined for chemisorption of oxygen by non-linear regression from TG in the range T min–T max with presumption of Fn kinetic model [11].
Determination of kinetic parameters of oxygen chemisorption on coal in temperature range T min–T max by direct non-linear regression from TG curve was performed in two steps. At first, all three parameters (reaction order n, activation energy E and frequency factor A) were optimized (data marked as calculation 1 in Table 2). Obtained values of reaction order n varied between 0.8 and 1.1 which indicates, that studied process can be described as first order reaction. The values of E and A were secondly recalculated with the presumption of n = 1 (first order kinetics, calculation 2 in Table 2) and these values are discussed below.
Obtained values of activation energy and frequency factor lie in close intervals (E = 81 ± 3 kJ mol−1, log10 A = 5.9 ± 0.3 s−1, 95% confidence intervals). Some outlying values were found in the case of the lowest initial masses of the coal samples—probably caused by the fact, that calculations were made from very low mass changes (below 0.1 mg). Regardless, activation energy and frequency factor are nearly unaffected by changing of sample mass, oxygen flow or crucible material used for thermoanalytical study of oxygen chemisorption on coal. Irrespective to their problematic physico-chemical meaning they are stable characteristics of the process.
It has to be noticed that tight linear relation exists between determined values of E and ln(A) (correlation coefficient r 2 = 0.999). This relation is known as kinetic compensation effect, and it probably exists as a mathematical consequence of the use of exponential Arrhenius equation for rate constant [12] and n-order kinetic model for complex process [13]. In fact, there is not any regular dependence of kinetic parameters on sample mass and other experimental conditions.
Mean value of calculated activation energy falls within the range of E determined for various bituminous coals from heat release rates up to 150 °C (≈50–100 kJ mol−1) [14]. On the other hand, it is relatively high in comparison with data published for low-temperature coal oxidation below 150 °C determined from the rate of oxygen consumption using gas chromatographic method (10–50 kJ mol−1) [15].
Heat of oxygen chemisorption from DSC curve
While the coal is heated in air or oxygen, the exothermic creation of oxygen containing surface complexes begins around 150 °C [8]. Study of coal interaction with oxygen by TG/DSC measurements enables to evaluate the heat evolved during the oxygen chemisorption process—parameter Q (kJ g−1, heat evolved per g of initial coal sample). However, the studied chemisorption proceeds mainly on the coal surface, and thus, it is not quite reasonable to relate heat to the mass of the whole coal sample. A solution offered by simultaneous TG/DSC measurement insists in relating the heat to the mass increase in the same temperature interval. Thus, parameter Q O (kJ g−1) as heat evolved per g of coal mass increase in the range T min–T max was determined.
Although the measured mass increase does not exactly represent the whole amount of chemisorbed oxygen (some of the surface oxygenated species decompose at the same time as other originate), it can be considered as an approximation. Parameter Q O then approximately represents heat amount evolved during chemisorption of 1 g of oxygen on the coal surface.
Parameter Q O was found to depend clearly on initial sample mass (Fig. 4), the dependence being different for crucible materials used. Continuous decrease of Q O with increasing mass was confirmed in α-Al2O3 crucibles while increase of that for Pt/Rh crucibles appears. Moreover, Fig. 4 indicates that values of parameter Q O tend to the same value (≈ 50 kJ g−1) irrespective of the crucible material. This “limiting” value is in agreement with our previous results [16] obtained for coals with different granularity, and it approximately agrees with the heat of formation of carbonyl group (732 kJ mol−1, 46 kJ g−1, related to oxygen mass). Thus, carbonyl groups can be expected as main product of oxygen chemisorption on coal.
Heat evolved during chemisorption stage related to the coal mass increase as a dependence on experimental conditions. Empty symbols represent crucibles from α-Al2O3 with oxygen flow 20 resp. 200 cm3 min−1 (○ resp. ◊), black symbols correspond to crucibles from Pt–Rh alloy with oxygen flow 20 resp. 200 cm3 min−1 (● resp. ◆)
Different dependencies of Q O on sample mass are probably caused by differing thermal properties of both materials (thermal conductivity and capacitance). Pt/Rh crucible can act as a “cooler” enabling removal of a part of heat evolved in the crucible out of the sensor. It can lead to nearly zero value of measured Q (or Q O) for very small samples with very small exothermic effect, but the “cooling” effect becomes insignificant for larger samples. On the other hand, α-alumina has properties of thermal insulator keeping the evolved heat in the range of DSC sensor and giving higher values of Q (in comparison with Pt/Rh crucible) especially for small sample mass. The effects should be avoided by appropriate calibration of apparatus. However, the standard calibration procedures (used also in the study) are based on measurement of endothermic changes of standards (melting heat and/or decomposition heat), and the absolute amount of the heat measured during the calibration being much higher than that evolved during the oxygen chemisorption on coal. Simultaneously, the rate of heat consumption during calibration differs from heat evolution rate accompanying measured process.
Conclusions
Evaluation of series of thermoanalytical measurements of coal low-temperature oxidation leads to the following conclusions about the effect of experimental condition on parameters derived from TG-DSC.
-
The effect of gas (oxygen) flow is negligible (or hidden in common experimental errors) on any of the parameters. No extreme care is necessary for setting the gas flow in the wide range 20–200 cm3 min−1.
-
Material of crucibles does not influence parameters derived from TG, but it affects reaction heat Q O derived from DSC considerably. The use of α-Al2O3 leads to higher measured values of Q O in comparison with Pt–Rh crucible. The difference between Q O becomes smaller with increasing sample mass.
-
The initial mass of the sample taken for analysis does not affect kinetic parameters determined from TG (activation energy and frequency factor) and temperature of beginning of coal combustion (T max). Other assessed parameters depend on the sample mass but with increasing sample mass the effect becomes also smaller.
As a result, the use of higher sample mass (more than 10–15 mg) can be recommended for thermoanalytical investigations of coal oxidation at low-temperature range (ambient—cca 300 °C) although it is in opposition with recommendations for TA studying of combustion processes at high temperatures when small samples are generally required.
References
Kök MV. Temperature-controlled combustion and kinetics of different rank coal samples. J Therm Anal Cal. 2005;79:175–80.
Ozbas KE, Kök MV, Hicyilmaz C. DSC study of the combustion properties of Turkish coals. J Therm Anal Cal. 2003;71:849–56.
Kök MV. An investigation into the combustion curves of lignites. J Therm Anal Cal. 2001;64:1319–23.
Oreshko WF. On the oxidation of coals with different degree of metamorphosis. Izvest Akad Nauk SSSR, Otdel Tekh Nauk. 1951;7:1031–40. (in Russian).
Wang H, Dlugogorski BZ, Kennedy EM. Coal oxidation at low temperatures: oxygen consumption, oxidation products, reaction mechanism and kinetic modeling. Prog Energy Combust Sci. 2003;29:487–513.
Sen R, Srivastava SK, Singh MM. Aerial oxidation of coal-analytical methods, instrumental techniques and test methods: A survey. Indian J Chem Technol. 2009;16:103–35.
Kök MV. Recent developments in the application of thermal analysis techniques in fossil fuels. J Therm Anal Cal. 2008;91:763–73.
Li XR, Koseki H, Iwata Y. A study on spontaneous ignition of bituminous coal. Thermal Sci. 2009;13:105–12.
Ceylan K, Karaca H, Önal Y. Thermogravimetric analysis of pretreated Turkish lignites. Fuel. 1999;78:1109–16.
Feng B, Bhatia SK. On the validity of thermogravimetric determination of carbon gasification kinetics. Chem Eng Sci. 2002;57:2907–20.
Slovák V. Determination of kinetic parameters by direct non-linear regression from TG curves. Thermochim Acta. 2001;372:175–82.
Koga N, Šesták J. Kinetic compensation effect as a mathematical consequence of the exponential rate-constant. Thermochim Acta. 1991;182:201–8.
Koga N, Šesták J. Further aspects of the kinetic compensation effect. J Therm Anal Cal. 1991;37:1103–8.
Jones JC, Henderson KP, Littlefair J, Rennie S. Kinetic parameters of oxidation of coals by heat-release measurement and their relevance to self-heating tests. Fuel. 1998;77:19–22.
Taraba B, Čáp K. Investigations of coal substance by gas chromatography. Uhlí. 1985;33:278–80. (in Czech).
Slovák V, Taraba B. The effect of gas flow and granularity on the chemisorption stage of coal oxidation. Chem listy. 2008;102:742. (in Czech).
Acknowledgements
This study was supported by Czech Science Foundation, project No. 105/06/0630.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Slovák, V., Taraba, B. Effect of experimental conditions on parameters derived from TG-DSC measurements of low-temperature oxidation of coal. J Therm Anal Calorim 101, 641–646 (2010). https://doi.org/10.1007/s10973-010-0878-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-010-0878-6