Abstract
In this article, synthesis, characterization, and thermal properties of diacrylic/divinylbenzene copolymers based on the new aromatic tetrafunctional acrylate monomers are presented. The new monomers were generated by treatment of epoxides derived from various aromatic diols: naphthalene-2,3-diol (NAF), biphenyl-4,4′-diol (BIF), bis(4-hydroxyphenyl)methanone (BEP) or 4,4′-thiodiphenol (BES), and epichlorohydrin with acrylic acid. The addition reaction was carried out by a ratio of 0.5 mol of suitable epoxy derivative and 1 mol of acrylic acid in the presence of 0.7 wt% of triethylbenzylammonia chloride (TEBAC) as a catalyst and 0.045 wt% of hydroquinone as a polymerization inhibitor. The chemical structure of the prepared acrylate monomers was confirmed by 13C NMR and GC MS spectra. The emulsion–suspension polymerization of acrylate monomers with divinylbenzene (DVB) in the presence of pore-forming diluents (toluene + decan-1-ol) allowed obtaining microspheres containing pendant functional groups (hydroxyl groups). This process was carried out at constant mol ratio of acrylate monomers: DVB (1:1), and constant volume ratio of pore-forming diluents to monomers (1:1). The different concentrations of toluene in the mixture with decan-1-ol were used for qualifying the effect of the diluents on the microsphere characteristics. The influence of synthesis’s parameters on the properties of copolymer beads, e.g., pore size and surface area by BET method, the surface texture by AFM, swelling behavior in polar and non-polar solvents as well as thermal stability by differential scanning calorimetry (DSC), and thermogravimetric analysis (TG) was studied and discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Synthetic polymers play a very important role in everyday life as well as in different branches of industry. They exist throughout the world as materials ranging from nylon fibers to reinforced composites. Functional polymer networks have gained a great importance in many fields of science and industrial applications due to the variety of alternative modifications of their chemical and physical properties [1–4]. In recent years, polymeric materials with porous structure are finding ever increasing applications in various fields. They are applied as precursors for the preparation of various types of ion exchange resins, inert supports for chromatographic purposes, supports for classical catalysts, and membranes. They are usually used in chromatography, biotechnology, optics, electronics, and chemical technology [5–10]. Porous polymer beads have been prepared by copolymerization of vinyl monomers and cross-linking agents such as divinylbenzene in the presence of an inert diluents (porogens) by different methods of polymerizations: suspension, emulsion, dispersion, emulsion–suspension, etc. [11, 12]. The various factors, e.g., temperature, amount of cross-linking monomer and initiator, the type of vinyl monomers, composition of the porogenic solvents influence on porous structure, mechanical strength, thermal stability, and resistance to solvent absorption of beads [13]. The most popular macroporous copolymers based on styrene/divinylbenzene are hydrophobic materials which are characterized by insufficient exchange rates in aqueous media [14]. For application in aqueous system, less hydrophobic polymeric materials are desirable. Hydroxyacrylate or meth(acrylate) monomers or resins represent an important group of less hydrophobic materials. Copolymerization of multifunctional (meth)acrylate monomers with vinyl monomer allows to produce highly cross-linked polymers with high thermal stability, porous structure, mechanical strength, and resistance to solvent absorption due to the presence of hydrophilic groups in the structure of the obtained materials [15–24]. The meth(acrylate) monomers are frequently produced during the esterification process of epoxide compounds or resins with acrylic or methacrylic acid in the presence of a acidic or basic type catalyst or transition metal complexes [25]. Recently, many such hydrophilic, porous materials have received a great amount of attention due to their unique properties suitable for applications in biomedicine- or polymer-supported reactions using enzymes.
In this article, synthesis, characterization, and thermal properties of porous diacrylic/divinylbenzene copolymers are presented. They were produced during emulsion–suspension polymerization of new aromatic tetrafunctional acrylate monomers derivatives of various diols: naphthalene-2,3-diol (NAF), biphenyl-4,4′-diol (BIF), bis(4-hydroxyphenyl)methanone (BEP), or 4,4′-thiodiphenol (BES) with divinylbenzene (DVB) in the presence of pore-forming diluents (toluene + decan-1-ol). This preparation method allowed obtaining more hydrophilic porous microspheres containing ester groups and pendant functional groups (hydroxyl groups). The influence of synthesis’s parameters on surface area, surface texture, swelling behavior in polar, and non-polar diluents as well as thermal stability was discussed.
Experimental
Materials
Epoxides derived from various aromatic diols: naphthalene-2,3-diol (NAF), biphenyl-4,4′-diol (BIF), bis(4-hydroxyphenyl)methanone (BEP) or 4,4′-thiodiphenol (BES), and epichlorohydrin (I step, Fig. 1) were used as basic reagents in the synthesis of the new acrylate monomers. Their preparation and properties were precisely described in our previous articles [23, 24].
Bis(2-ethylhexyl)-sulfosuccinate sodium salt (DAC,BP), decan-1-ol, tetrahydrofuran (THF), and acrylic acid (A) were purchased from Fluka AG (Buchs Switzerland). Bis(4-hydroxyphenyl)sulfide, α,α′-azoisobisbutyronitrile, and divinylbenzene (DVB) were obtained from Merck (Darmstadt, Germany). DVB was washed with 3% aqueous sodium hydroxide prior to use. Reagent grade acetone, methanol, chloroform, toluene, sodium hydroxide, potassium hydroxide, and hydroquinone were from POCh (Gliwice, Poland). Triethylbenzylammonia chloride (TEBAC) was prepared as per the procedure described by Mąkosza [26].
Preparation of new aromatic tetrafunctional acrylate monomers
The new aromatic tetrafunctional acrylate monomers were generated by treatment of epoxides derived from various aromatic diols:naphthalene-2,3-diol (NAF), biphenyl-4,4′-diol (BIF), bis(4-hydroxyphenyl)methanone (BEP) or 4,4′-thiodiphenol (BES), and epichlorohydrin (I step, Fig. 1) with acrylic acid (II step, Fig. 1). This process was performed in a three-necked round-bottomed flask (500 cm3) equipped with a thermometer, a mechanical stirrer, and a heating jacket in the temperature range of 80–100 °C. The reaction was carried out with a ratio of 0.5 mol of suitable epoxy derivative of aromatic diols and 1 mol of acrylic acid in the presence of 0.7 wt% of TEBAC as a catalyst, and 0.045 wt% of hydroquinone as a polymerization inhibitor. The reaction course extent was controlled by estimating the value of an acid number (defined as a number of mg KOH required for the titration of 1 g of a sample) of the reaction mixture. The drop in the value of an acid number below 3 mg KOH/g marked the indication of the process completion. In this way, the new, corresponding aromatic tetrafunctional acrylate monomer derivatives of various diols, such as NAF–DA, BIF–DA, BEP–DA, or BES–DA were prepared. Their respective chemical structures were confirmed by 13C NMR and GC MS methods.
Preparation of diacrylic/divinylbenzene copolymers
The copolymers in the form of microspheres were produced during the emulsion–suspension polymerization of new aromatic tetrafunctional acrylate monomers and DVB. First, 150 mL of redistilled water and 1.4 g of bis(2-ethylhexyl)sulfosuccinate sodium salt (surfactant) were placed in a three-necked flask equipped with a stirrer, a water condenser, and a thermometer and stirred for 1 h at 80 °C to obtain the aqueous medium. Then, 1.5 wt% of α,α′-azoisobisbutyronitrile (initiator), the mixture of pore-forming diluents (toluene and decan-1-ol), suitable acrylate monomer, and DVB were added to the aqueous medium forming a solution with stirring. The emulsion–suspension polymerization was performed at the constant mol ratio of acrylate monomer:DVB (1:1) at 80 °C for 18 h. The obtained microspheres (NAF.A–DVB, BIF.A–DVB, BEP.A–DVB, or BES.A–DVB) were thoroughly washed with distilled water, filtered out, dried, and extracted in a Soxhlet apparatus with boiling acetone and methanol.
Characterization
GC MS was made on a Thermo-Finnigan DSQ spectrometer (Finnigan, USA) connected to a gas chromatograph Trace GC-Ultra equipped with a fused-silica RTX-5 capillary column (20 m × 0.18 mm I.D., film thickness 0.20 μm). The conditions were as follows: injector PTV-split 1:20, program temperature 35–320 °C at the rate of 20 °C min−1, MS electron ionization at 70 eV, and temperature of ion volume 220 °C.
13C NMR spectra were recorded on a Brucker 300 MSL instrument (Brucker, Germany). Chemical shifts were referred to chloroform serving as an internal standard.
The specific surface areas were calculated by the BET method, assuming that the area of a single nitrogen molecule is 16.2 Å2. These determinations were made with the use of an adsorption analyzer ASAP 2405 (Micrometrics Inc., USA). The measurements of the surface properties of the copolymers were preceded by activation of the samples at 200 °C for 2 h.
The microspheres were also tested by an atomic force microscope (AFM), AFM Nanoscope III (Digital Instruments, USA), operating in the contact mode. The presented images contained 512 × 512 data points obtained within a few seconds. The typical force applied to obtain these images ranged from 1.0 to 100 nN.
The calorimetric measurements were carried out in the Netzsch DSC 204 calorimeter (Selb, Germany) operating in a dynamic mode. The dynamic scans were performed at a heating rate of 10 K min−1 at two temperature scans. First scan was performed from 20 °C to a maximum of 110 °C to remove any adsorb moisture. The second one was conducted between 20 and 500 °C under nitrogen atmosphere (30 mL min−1). The mass of the sample was ~10 mg. As a reference, an empty aluminum crucible was used. The glass transition temperature (T g), decomposition temperature (T d), and enthalpy of decomposition (ΔH d) were evaluated.
Thermogravimetric analysis (TG) was carried out on a Paulik and Erdey derivatograph (Budapest, Hungary) at a heating rate of 10 °C min−1 in air, in the temperature range of 20–1000 °C with the sample weight of 100 mg. As a reference, α-Al2O3 was used. The initial decomposition temperature (IDT), T 20%, and T 50% of weight loss, final decomposition temperature (T end), and temperature of the maximum rate of weight loss (T max) were determined.
Results and discussion
Characterization of new aromatic tetrafunctional acrylate monomers
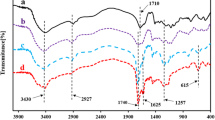
The theoretical chemical structure of prepared new aromatic tetrafunctional acrylate monomers: NAF–DA, BIF–DA, BEP–DA, or BES–DA is presented in Fig. 1. Their structure is confirmed by 13C NMR, Table 1, Fig. 2; and GC MS analyses, Fig. 3. The characteristic signals for carbon terminal of the double bond and more highly substituted ones as well as signals for the carbonyl groups, methylene groups, and secondary hydroxylic groups were found. It confirmed that the addition reaction of epoxide groups to the carboxylic ones was successful. No side reactions of the formed secondary hydroxyl groups with epoxy groups (etherification) or with acid groups (esterification) were observed. In addition, GC MS analysis of prepared monomers clearly showed the presence of molecular ions corresponding to the calculated molecular weights of NAF–DA (416 g mol−1), BIF–DA (442 g mol−1), BEP–DA (470 g mol−1), and BES–DA (474 g mol−1). The exemplary mass spectrum of BES–DA monomer is presented in Fig. 3.
Characterization of diacrylic/divinylbenzene copolymers
The copolymers were produced during the emulsion–suspension polymerization of new aromatic tetrafunctional acrylate monomers with DVB in the presence of pore-forming diluents (toluene + decan-1-ol). This process was performed at a constant mol ratio of acrylate monomers:DVB (1:1) and a constant volume ratio of pore-forming diluents to monomers (1:1). Different concentrations of toluene in the mixture with decan-1-ol were used, Table 2 to study the influence of organic solvents on the microsphere characteristic, their porous structure, and pore size distribution. The preparation of microspheres based on NAF–DA and BES–DA monomers for all the used concentrations of toluene with the mixture of decan-1-ol was observed, Table 2. However, probably owing to the poor dissolubility of solid BIF–DA and BEP–DA monomers in toluene, the formation of microspheres in decan-1-ol were only observed (synthesis no. 6, Table 2). The results presented in Tables 3 and 4, suggested that the surface characteristic and the pore structure of acrylic copolymers significantly depended on the chemical structure of monomers and the nature of the used solvents. The swellability coefficients (B) varied from 12 to 28% for all the polar solvents. The significantly higher values of B in polar solvents compared to those values in non-polar hexane were observed. Such behavior was probably due to the presence of polar pendant functional groups (hydroxyl groups) in copolymers. Moreover, the highest swelling and surface area but lower average pore diameter for BES.A–DVB copolymer compared with NAF.A–DVB was observed, Table 4. It was probably connected with the presence of macro- and micropores in the structure of BES.A–DVB copolymer. Higher elasticity of monomer containing sulfur caused larger penetration of porogens diluents during polymerization process resulting in the formation of more developed porous structure with a relatively broad pore size. The surface texture studies by AFM performed in addition confirmed that NAF.A–DVB and BES.A–DVB copolymers were characterized by a porous structure, Fig. 4. On the contrary, BIF.A–DVB and BEP.A–DVB copolymers were characterized by the lowest swelling properties. This characterization is connected with non-porous, faintly developed surface area which is clearly observed from AFM images.
Thermal properties of NAF.A–DVB, BIF.A–DVB, BEP.A–DVB and BES.A–DVB copolymers were studied by means of DSC and TG analyses. The DSC was performed in a nitrogen atmosphere with temperatures ranging from 20 to 500 °C. The TG was conducted in air from 20 to 1000 °C. The DSC curves of diacrylic/divinylbenzene copolymers are presented in Fig. 5. In addition, the characteristic temperatures: T g, T d, and the enthalpy of decomposition (ΔH d) are given in Table 5. DSC analysis showed similarity in thermal behavior for all the prepared copolymers. The characteristic, well-shaped calorimetric profile, revealing two non-distributed asymmetrical peaks was observed. The first, exothermic peak (T max1) starting at about 300 °C can be attributed to the additional double bond copolymerization process. The endothermic peaks at 421.5–434.4 °C with ΔH d values from 58.8 to 100.6 J g−1 corresponded with the thermal degradation of cross-linked microspheres. Moreover, the copolymers were characterized by rather high thermal stability, and no endothermic decomposition peak until 250–300 °C was observed. The TG analysis confirmed those observations. The initial decomposition temperatures (IDTs) for all copolymers were from 250 to 310 °C. The final decomposition temperatures (T end) were in the range of 780–940 °C. The DTG curves contained two separated degradation steps. The first decomposition peak was observed in the range of 250–390 °C with the maximum weight loss (T max1) at 360–375 °C. The second decomposition stage took place between 480 and 940 °C with T max2 at 565–575 °C, Table 6. The first decomposition peak could be associated with the ester bonds breakdown in copolymers, whereas the second one could be attributed to the total degradation of copolymers [27, 28].
Conclusions
The synthesis, characterization, and thermal properties of diacrylic/divinylbenzene copolymers based on the new aromatic tetrafunctional acrylate monomers were presented. The acrylate monomers were obtained by treatment of epoxides derived from various aromatic diols: naphthalene-2,3-diol (NAF), biphenyl-4,4′-diol (BIF), bis(4-hydroxyphenyl)methanone (BEP) or 4,4′-thiodiphenol (BES), and epichlorohydrin with acrylic acid. Their emulsion–suspension polymerization with DVB in the presence of pore-forming diluents (toluene + decan-1-ol) allowed obtaining more hydrophilic microspheres due to the presence of ester and hydroxyl groups in their structure. Based on the performed analyses, it was found that the parameters of the synthesis as well as the structure of initial acrylate monomers had a significant influence on the properties of final product. DSC and TG analyses indicated on high thermal stability of diacrylic/divinylbenzene copolymers up to 250–300 °C which was directly connected with their high cross-lined network structure.
References
Kotha A, Raman RC, Ponrathnam S, Shewale JG. Beaded reactive polymers, 1. Effect of synthesis variables on pore size and its distribution in beaded glycidyl methacrylate–divinylbenzene copolymers. React Funct Polym. 1996;28:227–33.
Chiu YY, Saito R, Lee LJ. Modification of unsaturated polyester resin for viscosity control. Polymer. 1996;37:2179–90.
Bicak N, Sherrington D. Mercury sorption by “non-functional” crosslinked polyacrylamides. React Funct Polym. 1995;27:155–61.
Ciesińska W, Zieliński J, Brzozowska T. Thermal treatment of pitch-polymer blends. J Therm Anal Calorim. 2008;95:193–6.
Ishizu K. Synthesis and structural ordering of core-shell polymer microspheres. Prog Polym Sci. 1998;23:1383–408.
Kawaguci H. Functional polymer microspheres. Prog Polym Sci. 2000;25:1171–210.
Zhang X-Z, Lewis PJ, Chu C-C. Fabrication and characterization of a smart drug delivery system: microsphere in hydrożel. Biomaterials. 2005;26:3299–309.
Xiao X, Zhuo R, Xu J, Chen L. Effects of reaction temperature and reaction time on positive thermosensivity of microspheres with poly(acrylamide)/poly(acrylic acid) IPN shells. Eur Polym J. 2006;42:473–8.
Stanek LG, Hellmann SM, Gleason WB. Preparation of monodisperse PMMA microspheres using 2-vinyl-4, 4′-dimethylazlactone as a spacer stabilizer. Colloid Polym Sci. 2006;284:586–95.
Luciani A, Coccoli V, Orsi S, Ambrosio L, Netti PA. PCL microspheres based functional scaffolds by bottom-up approach with predefined microstructural properties and release profiles. Biomaterials. 2008;29:4800–7.
Svec F, Fréchet JMJ. Temperature, a simple and efficient tool for the control of pore size distribution in macroporous polymers. Macromolecules. 1995;28:7580–2.
Sipos P, Szabo A, Erös I, Szabó-Rėwėsz P. A DSC and Raman spectroscopic study of microspheres prepared with polar cosolvents by different techniques. J Therm Anal Calorim. 2008;94:109–18.
Bisjak CP, Lubbad SH, Trojer L, Bonn GK. Novel monolithic poly(phenyl acrylate-co-1,4-phenylene diacrylate) capillary columns for biopolymer chromatography. J Chromatogr A. 2007;1147:46–52.
Rohr T, Knaus S, Sherrigton DC, Gruber H. Synthesis of sugar-containing hydrophilic porous polymer supports via suspension polymerization. Acta Polym. 1999;50:286–92.
Hradil J, Wojaczyńska M, Svec F, Kolarz BN. Sorption of phenols on macroporous methacrylate copolymers containing ethyleneamine groups. React Polym. 1986;4:277–83.
Narasimhaswamy T, Reddy BSR. Phenyl acrylates and divinylbenzene cross-linked copolymers as basic novel supports: synthesis and characterization. J Appl Polym Sci. 1991;43:1645–57.
Azanova VV, Hradil J. Sorption properties of macroporous and hypercrosslinked copolymers. React Funct Polym. 1999;41:163–75.
Smigol V, Svec F. Synthesis and properties of uniform beads based on macroporous copolymer glycidyl methacrylate-ethylene dimethacrylate: a way to improve separation media for HPLC. J Appl Polym Sci. 1992;46:1439–48.
Smigol V, Svec F. Preparation and properties of uniform beads based on macroporous glycidyl methacrylate-ethylene dimethacrylate copolymers: use of chain transfer agent for control of pore-size distribution. J Appl Polym Sci. 1993;48:2033–9.
Gawdzik B, Gawdzik J, Czerwińska-Bil U. Copolymer of di(methacryloyloxymethyl) naphthalene and divinylbenzene as a column packing for high-performance liquid chromatography. Chromatographia. 1988;26:399–407.
Gawdzik B, Osypiuk J. Reversed-phase high-performance liquid chromatography on porous copolymers of different chemical structure. J Chromatogr A. 2000;898:13–21.
Gawdzik B, Podkościelna B. An influence of diluent composition on the porous structure of 4,4′-diphenyl dimethacrylate–divinylbenzene copolymers. Ann UMCS. 2005;LX:282–95.
Gawdzik B, Podkościelna B, Bartnicki A. Synthesis, structure and properties of new methacrylic derivatives of naphthalene-2,3-diol. J Appl Polym Sci. 2006;102:1886–95.
Podkościelna B, Gawdzik B, Bartnicki A. Use of a new methacrylic monomer, 4, 4′-di(2-hydroxy-3-methacryloyloxypropoxy)benzophenone in the synthesis of porous microspheres. J Polym Sci A Polym Chem. 2006;44:7014–26.
Bukowska A, Bukowski W, Galina H, Sztycharz A. The kinetics of the reaction of methacrylic acid with Epidian 6. Polimery. 2000;45:109–12.
Mąkosza M. Synteza organiczna. Warsaw: PWN; 1972.
Worzakowska M. Thermal analysis of polyesters containing oxirane groups. J Therm Anal Calorim. 2008;93:799–803.
Worzakowska M. The influence of chemical modification of unsaturated polyesters on viscoelastic properties and thermal behavior of styrene copolymers. J Therm Anal Calorim. 2009;96:235–41.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Podkościelna, B., Worzakowska, M. Synthesis, characterization, and thermal properties of diacrylic/divinylbenzene copolymers. J Therm Anal Calorim 101, 235–241 (2010). https://doi.org/10.1007/s10973-009-0574-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-009-0574-6