Abstract
Primaquine (PQ) is the drug of choice for the radical cure of Plasmodium vivax malaria, and currently being administered in solid dosage form. In this study, the compatibility studies were carried out using differential scanning calorimetry (DSC), thermogravimetry (TG), and fourier transformed infrared (FT-IR). Non-isothermal and isothermal methods were employed to investigate kinetic parameters under nitrogen and air atmospheres using TG. The DSC investigations obtained by physical mixtures showed slight alterations in the melting temperatures of PQ with some excipients. The FT-IR confirmed the possible interactions obtained by DSC for the physical mixtures with PQ and lactose, magnesium stearate and mannitol. The results showed that the thermal decomposition followed a zero order kinetic in both atmospheres in non-isothermal method. The activation energy in both methods using nitrogen atmosphere was similar, and in air atmosphere the activation energy decreased.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
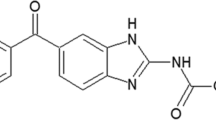
Primaquine (PQ) (Fig. 1) that corresponds to 8-aminoquinoline is used to prevent relapses of malaria and in prophylaxis of individuals returning from malaria’s areas. It is the drug of choice for the radical cure of Plasmodium vivax malaria, although has serious side effects, for example, acute hemolysis in patients with glucose 6-phosphate dehydrogenase deficiency, methemoglobinemia, and severe gastrointestinal disturbances [1, 2]. Thus, the development of controlled release dosage form has been investigated to improve the treatment efficacy and reduce the limitations of existing therapies [3–6].
The successful formulation of a stable and effective solid dosage form depends on careful excipients selection. The most drugs intended for oral administration requires formulation with excipients to allow adequate administration, to facilitate the product manufacture, to increase the formulation stability and for aesthetic reasons [7, 8].
The thermal analysis is a routine method applied for drugs characterization and is useful in the pre-formulation stage in the development of solid dosage forms [9–14].
Thermogravimetry (TG) and differential scanning calorimetry (DSC) techniques gives important information about the physical properties, kinetic analysis, polymorphic forms, and stability of materials, as well as to assess its compatibility with excipients [15–19] during processing and storage [9, 11] as a function of temperature.
The thermal decomposition of drugs is interesting to predict the degradation rates at marketing temperatures from data collected on accelerated processes that are studied at elevated temperatures. The temperature may increase the chemical reactions, providing sufficient energy (activation energy) required to break chemical bonds and starts the decomposition process [20, 21].
The main purpose of the present study was to evaluate the compatibility of PQ with common pharmaceutical excipients used in solid dosage forms as well to investigate the influence of different methods (isothermal and non-isothermal) under nitrogen and air atmosphere involved in PQ degradation.
Experimental
Materials
The PQ raw material was kindly donated by Fundação Oswaldo Cruz/Far-Manguinhos (state purity: 98.5%). The excipients used were hydroxypropylmethylcellulose (HPMC) of viscosity grade K15M (Methocel K15 M Premium), acryl-eze®, microcrystalline cellulose (Avicel Ph-102), starch, magnesium stearate, lactose, colloidal silicon dioxide (Aerosil, Galena), poly ethylene oxide (POE) of molecular weight grade 8.106 Da (Sigma–Aldrich), talc, stearic acid, tribasic calcium phosphate, glyceryl monostearate, mannitol, povidone, sodium stearyl fumarate, and stearyl alcohol.
Methods
Differential scanning calorimetry analysis (DSC)
The DSC curves were obtained in a DSC-60 cell (Shimadzu) using aluminum crucibles with about 2 mg of samples, under dynamic nitrogen atmosphere (50 mL min−1). The temperature range was from 298 K to 873 K at heating rate of 283 K min−1. An empty aluminum pan was used as reference. DSC analysis have been performed using sample of PQ, single excipients, binary physical mixtures formed by PQ and only one excipient 1:1 (w/w) with all excipients samples. The DSC cell was calibrated with indium (melting point: 429.6 K; ∆H fus = 28.54 J g−1) and zinc (melting point: 692.6 K).
Thermogravimetric analysis (TG)
The TG experiments were measured on Shimadzu thermobalance TGA – 50 in temperature range from 298 K to 873 K, using platinum crucibles with approximately 4 mg of sample, under dynamic N2 atmosphere (50 mL min−1). TG analysis have been using sample of PQ, single excipients, binary mixtures formed by PQ and only one excipient 1:1 (w/w) with all excipients samples. The equipment was previously calibrated with calcium oxalate standard.
Fourier transformed infrared spectroscopy (FT-IR)
FT-IR spectra was recorded on a Perkin-Elmer Model 1600 apparatus using KBr discs in the range of 4000–400 cm−1. The sample of PQ and the excipients (1:1) used were lactose, magnesium stearate, stearyl alcohol, sodium stearyl fumarate, glyceryl monostearate, mannitol, and stearic acid.
Kinetic studies
The non-isothermal kinetic study was performed by application of Ozawa method [22]. In dynamic experiments, the heating rates used were 275.5, 278, 283, 288, and 293 K min−1 to target temperature of 873 K under nitrogen and air atmosphere with the flow rate of 50 mL min−1.
The isothermal studies were evaluated by holding different samples isothermally at 493, 488, 483, 478 and 473 K under nitrogen and air atmosphere at flow rate of 50 mL min−1. The isothermal holding was monitored based on the time to mass loss of 8% degradation and the experimental data were treated applying linear regression analysis. The heating rate used was 283 K min−1.
Results and discussion
The DSC curve of PQ shows a single sharp endothermic peak corresponding to the melting event in the range between 474 and 481 K (T peak = 479.57 K and ∆H fusion = −83.93 J g−1) [23, 24], followed by decomposition process. The result was confirmed by TG analysis and can be better visualized by applying the first derivative (DTG), which the thermal decomposition process occurs in three stages in the following temperature range and mass loss: 481–531 K (∆m = 3.3%), 531–643 K (∆m = 33%), and 580–1173 K (∆m = 38.6%) (Fig. 2).
Compatibility studies
The selection of adequate excipients for the formulation should be based on the characteristic of the drug and its compatibility and stability with other components.
The DSC and TG curves of binary mixture (1:1) of drug and the following excipients: HPMC, colloidal silicon dioxide, talc, starch, tribasic calcium phosphate, acryl-eze®, POE, povidone, and microcrystalline cellulose are showed in Figs. 3, 4, 5 and 6.
The thermal profiles of the mixture can be considered as a superposition of the curves of the PQ and excipients. The DSC and TG curves showed an endothermic peak corresponding to the PQ’s melting point followed by exothermic events characteristic of decomposition process. The thermal behavior of PQ was not modified in the binary mixtures, suggesting no interaction with these excipients.
The compatibility study of the drug and the following excipients: lactose, magnesium stearate, stearyl alcohol, sodium stearyl fumarate, glyceryl monostearate, mannitol and stearic acid are showed in Figs. 7 and 8. The DSC curves demonstrated differences in the thermal profile of the PQ, such as absence of drug’s melting event. The TG curves demonstrated that excipients influence the decomposition process of the PQ by displacing the T onset of the first mass loss event at a lower temperature than the isolated drug.
The interactions between PQ and lactose might be physical in nature which can be attributed to similar melting’s temperature ranges (478–488 K). In this binary mixture, the melting point of the drug was decreased from 481 to 393 K. In fact, similar results were observed for other amines and amides, such as glimepiride [9], glipizide [25], and glibenclamide [26].
Differences in the thermal curves of other drugs with magnesium stearate were described by others authors [14, 26, 27].
The drug’s endothermic peak was extended in the DSC curves with the excipients stearyl alcohol, sodium stearyl fumarate and stearic acid, which were attributed to PQ dissolution in the melted excipient.
The TG and DSC dates obtained in the compatibility studies were demonstrated in Table 1.
The FT-IR spectroscopy was used as supplementary technique in order to investigate the possible chemical interaction between drug–excipient and to confirm the results obtained by the thermal analysis [28].
The FT-IR spectrums obtained with the PQ and the excipients sodium stearyl fumarate, glyceryl monostearate, stearic acid, stearyl alcohol (Fig. 9) demonstrated that the characteristic PQ stretching bands were maintained, indicating that no occurs chemical interactions between PQ and these compounds.
In Fig. 10 the FT-IR spectrums of physical mixtures between PQ and lactose, magnesium stearate and mannitol can be observed. The band in 1050 cm−1, characteristic of axial deformation C–O of PQ, expanded or moved about 10 cm−1 in physical mixtures of drug and these excipients, indicating an intermolecular links and possible chemical incompatibility. The FT-IR spectrums for the physical mixture between PQ/lactose and PQ/mannitol suggest chemical interactions. The amine group presented in PQ can react chemically with OH group presented in these excipients by hydrogen bonds.
Kinetic studies of PQ
One of the main purposes of kinetic analysis of solid decomposition is to determine reaction mechanisms. The results obtained in the PQ kinetics investigation using isothermal and non-isothermal methods were similar. However, a rather difference was observed in the activation energy obtained under nitrogen and synthetic air demonstrated that the atmosphere involved in assay presented influence on the data.
The non-isothermal kinetic data were determined by plotting mass loss versus temperature of five TG curves at different heating rates in both atmospheres. The results demonstrate that TG curves in nitrogen and air atmosphere are shifted for higher temperatures when heating rates increases with a good correlation in Ozawas plot at five heating rates in nitrogen (Fig. 11) and a low correlation in air (Fig. 12).
The order of reaction and the activation energy (E a) of process were determined by Ozawa’s method in which plots slope of log heating rate versus 1/T [22]. The kinetics parameters obtained in non-isothermal method for the first stage of thermal decomposition was around 481–531 K (Table 2).
Under isothermal conditions, the curves (Fig. 13 and 14) showed that the mass loss variation depends on the temperature. As higher the temperature in the assay the smaller will be the time necessary for the same mass loss to occur. The curves were used to obtain the graphic of lnt versus 1/T (K − 1) at a constant conversion level. The regression linear equation, correlation coefficient, and E a were represented in Table 3.
The comparison between isothermal and non-isothermal methods in nitrogen atmosphere shows good agreement for both values of activation energy. The decomposition kinetics for both methods occurs in constant rate, zero order, and it is independent of the reactants concentrations.
In the other hand, using air atmosphere in both methods the activation energy values was lower than nitrogen. In fact, PQ has a chemical group that suffers oxidation, therefore, under synthetic air atmosphere undergoes acceleration in the decomposition process.
Conclusions
In the compatibility studies, the results demonstrated the applicability of DSC as a fast screening tool for excipients at the early stages of a preformulation process. The FT-IR completes the DSC studies. The excipients, lactose, magnesium stearate, and mannitol, showed a possible chemical incompatibility. The present work will contribute to select the appropriate excipients in order to formulate a safe and stable PQ solid dosage form.
The thermoanalysis confirmed to be a suitable technique to evaluate the order of decomposition process of PQ. The decomposition kinetics for non-isothermal and isothermal methods occurs in constant rate, zero order, and is independent of the concentration of the reactants. Therefore, under air atmosphere in both methods the activation energy values was lower than nitrogen, demonstrating that the PQ has a chemical group that suffers oxidation.
References
Baird JK, Fryauff DJ, Hoffman SL. Primaquine for prevention of malaria in travelers. Clin Infect Dis. 2003;37:1659–67.
Vale N, Moreira R, Gomes P. Primaquine revisited six decades after its discovery. Eur J Med Chem. 2009;44:937–53.
Bhadra A, Yadav K, Bhadra S, Jain NK. Glycodendrimeric nanoparticulate carriers of primaquine phosphate for liver targeting. Int J Pharm. 2005;295:221–33.
Gaspar R, Prat V, Roland M. Nanoparticles of polyisohexylcyanoacrylate (PIHCA) as carriers of primaquine: formulation, physico-chemical characterization and acute toxicity. Int J Pharm. 1991;68:111–9.
Green MD, D’Souza MJ, Holbrook JM, Wirtz RA. In vitro and in vivo evaluation of albumin-encapsulated primaquine diphosphate prepared by nebulization into heated oil. J Microencapsul. 2004;21:433–44.
Mayorga P, Puisieux F, Couarraze G. Formulation study of a transdermal delivery system of primaquine. Int J Pharm. 1996;132:71–9.
Jackson K, Young D, Pant S. Drug-excipient interaction and their affect on absorption. Res Focus. 2000;3:336–45.
Mura P, Faucci MT, Manderioli A, Bramanti G, Ceccarelli L. Compatibility study between ibuproxam and pharmaceutical excipients using differential scanning calorimetry, hot-stage microscopy and scanning electron microscopy. J Pharm Biomed Anal. 1998;18:151–63.
Cides LCS, Araújo AAS, Santos-Filho M, Matos JR. Thermal behavior, compatibility study and decomposition kinetics of glimepiride under isothermal and non-isothermal conditions. J Therm Anal Calorim. 2006;84:441–5.
Nunes RS, Semaan FS, Riga AT, Cavalheiro ETG. Thermal behavior of verapamil hydrocholide and its association with excipients. J Therm Anal Calorim; 2009. doi:10.1007/s10973-009-0072-x.
Giron D. Contribution of thermal methods and related techniques to the rational development of pharmaceuticals, Part 1. PSTT. 1998;1:191–9.
Giron D. Contribution of thermal methods and related techniques to the rational development of pharmaceuticals, Part 2. PSTT. 1998;1:262–8.
Silva MAS, Kelmann RG, Foppa T, Cruz AP, Bertol CD, Sartori T, et al. Thermoanalytical study of fluoxetine hydrochloride. J Therm Anal Calorim. 2007;87:463–7.
Stulzer HK, Rodrigues PO, Cardoso TM, Matos JSR, Silva MAS. Compatibility studies between captopril and pharmaceutical excipients used in tablets formulations. J Therm Anal Calorim. 2008;91:323–8.
Sashina ES, Janowska G, Zaborski M, Vnuchkin AV. Compatibility of fibroin/chitosan and fibroin/cellulose blends studied by thermal analysis. J Therm Anal Calorim. 2007;89:887–91.
Gennaro AR. Remington’s the pharmaceutical sciences and practice of pharmacy. Philadelphia: Lippincott, Williams & Wilkins; 2004.
Mura P, Gratteri P, Faucci MT. Compatibility studies of multicomponent tablet formulations DSC and experimental mixture design. J Therm Anal Calorim. 2002;68:541–51.
Kiss D, Zelkó R, Novak CS, Éhen ZS. Application of DSC and NIRS to study the compatibility of metronidazole with different pharmaceutical excipients. J Therm Anal Calorim. 2006;84:447–51.
Bruni G, Amici L, Berbenni V, Marini A, Orlandi A. Drug-excipient compatibility studies: search of interaction indicators. J Therm Anal Calorim. 2002;68:561–73.
Felix FS, Cides LCS, Angnes L, Matos JR. Thermal behavior study and decomposition kinetics of salbutamol under isothermal and non-isothermal conditions. J Therm Anal Calorim. 2009;95:877–80.
Burnham L, Dollimore D, Alexander K. Kinetic study of the drug acetazolamide using thermogravimetry. Thermochim Acta. 2002;392:127–33.
Ozawa T. Thermal analysis—review and prospect. Thermochim Acta. 2000;355:35–42.
Al-Badr AA. Primaquine diphosphate: comprehensive profile. Profiles Drug Subst Excipients Relat Methodol. 2005;32:153–207.
British Pharmacopoeia. 3ed. London; 1999.
Verma RK, Garg S. Selection of excipients for extended release formulations of glipizide through drug-excipient compatibility testing. J Pharm Biomed Anal. 2005;38:633–44.
Oliveira GGG, Ferraz HFG, Matos JSR. Thermoanalytical study of glibenclamide and excipients. J Therm Anal Calorim. 2005;79:267–70.
Lerdkanchanaporn S, Dollimore D, Alexander KS. A thermogravimetric study of ascorbic acid and its excipients in pharmaceutical formulations. Thermochimica Acta. 1996;284:115–26.
Bugay DE. Characterization of the solid-state: 2. Spectroscopic techniques. Adv Drug Deliv Rev. 2001;48:43–65.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bertol, C.D., Cruz, A.P., Stulzer, H.K. et al. Thermal decomposition kinetics and compatibility studies of primaquine under isothermal and non-isothermal conditions. J Therm Anal Calorim 102, 187–192 (2010). https://doi.org/10.1007/s10973-009-0540-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-009-0540-3