Abstract
In the previous study, it was observed that the stability of nitrocellulose (NC) cannot be determined by thermal analyses such as differential scanning calorimetry (DSC) at heating rates of 1–10 K/min. This was because the thermal curves of NC samples with different stabilities could not be distinguished from one another. In this study, we explain why such thermal analyses cannot be used to evaluate the thermal stability of NC and identify the conditions under which thermal analyses can be used for this purpose. We investigated the effect of heating rate on the thermal behavior of pure NC and NC stabilized with diphenylamine (DPA) or akarditeII (AKII), which is a conventional stabilizer, by using the heat flux calorimeter (C80). At high heating rates (0.2–0.3 K/min), only single exothermic peak was observed in the thermal curves of both pure NC and NC/DPA and the thermal curve of pure NC was practically similar to that of NC/DPA. At low heating rate (0.02 K/min), two exothermic peaks were observed for both pure NC and NC/DPA. The heat amount of the first peak depended on the partial pressure of O2 in the atmosphere. The first peak in the thermal curve of NC/DPA was slightly suppressed as compared to that of pure NC. These results indicate that the stability of NC probably depends on the first exothermic peak that represents oxidation of NC by atmospheric O2. From this, on the thermal analyses at high heating rates, thermal curves of pure NC and NC/DPA could not be distinguished from one another. This is because the decomposition of NC itself occurs in the second exothermic peak before the oxidation of NC by atmospheric O2 in the first peak, which is attributed to the stability of NC. The results of the thermal analyses under isothermal conditions at 393 K in an O2 atmosphere revealed that the induction period of NC/DPA and NC/AKII was longer than that of pure NC. From these results, it is speculated that the stability of NC can be evaluated by thermal analyses carried out under O2-rich conditions at low heating rates.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nitrocellulose (NC) is widely used as an ingredient in propellants, explosives, fireworks, and gas generators, although it is capable of igniting spontaneously [1, 2]. The tendency of NC to undergo spontaneous ignition has been the cause of serious accidents in the recent years [3].
The mechanism underlying the spontaneous ignition of NC has been investigated extensively. It is widely known that the spontaneous ignition of NC is due to its reaction with NO2 released by bond scissions, and the hydrolysis of O–NO2 bond [1, 2]. Recently, we proposed that NO2 contributes to the initiation process of the spontaneous ignition of NC, and that atmospheric O2 favors the heat release [4–6]. In order to prevent the spontaneous ignition of NC, stabilizers such as diphenylamine (DPA) and akarditeII (AKII) are used in industries. It has been reported that the stabilizer reacts easily with NO2 generated by bond scission in NC to produce nitro and nitroso derivatives, thereby terminating the exothermic reaction [1, 2, 7, 8]. Moreover, it has been reported that DPA reacts with the other radical species such as alkoxy or peroxy radical as well [9, 10].
The ABEL heat test [11], which is used to estimate the amount of NO2 in smokeless powders, is a mandatory stability test in Japan. Although this test is simple and extremely useful for the determination of the stability of NC, it has certain disadvantages [12]. For example, since this test is based on visual inspection, results may vary with the analyst. Moreover, the ABEL heat test cannot be used to evaluate the stability of NC under certain conditions [13]. Hence, we concluded that it is preferable to evaluate the stability of NC by NO2 measurements and other methods such as thermal analyses. However, previous studies [14, 15] have reported that common thermal analyses such as temperature differential scanning calorimetry (DSC) at heating rates of 1–10 K/min cannot be used to evaluate the stability of NC because the thermal curves of NC samples with different stabilities are indistinguishable from one another.
The purpose of this study is to identify the reason for the failure of thermal analyses to evaluate the thermal stability of NC; further, the conditions under which thermal analyses can be used to estimate the stability of NC are presented herein. For this purpose, we assumed that the heating rate at elevated temperatures contributes to the phenomenon. On the basis of this assumption, we investigated the effect of heating rate on the thermal behavior of NC samples with different stabilities.
Experimental
NC in 2-propanol (nitrogen content: 12 mass%; Sigma-Aldrich Corp.) was dried under vacuum at room temperature for 3–4 days. Particles with diameters of 75–106 μm were separated by sieving and again dried under the same conditions for 1–2 days. The NC powder thus obtained was used as the experimental sample. NC/DPA (Sigma-Aldrich Corp.) and NC/AKII (provided by NOF Corp.) were also used. Those additives were used after crushing its particles.
We used 50 mg of pure NC and 50 mg of NC mixed with 1 mg of the stabilizer for the thermal analysis; the samples were placed in a 4-cm3 steel ampoule. The air in the ampoule was removed under vacuum and replaced by dry air or O2 (Suzuki Shokan Corp., Ltd.). This process was repeated 4–5 times in order to ensure complete replacement of atmospheric air. The sample was then stored in a C80 calorimeter (Setaram Instrumentation) for simultaneous investigation of the thermal and pressure behaviors of the sample.
Results and discussion
Thermal behavior at high heating rates
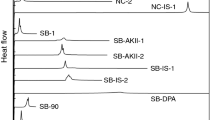
The thermal behaviors of pure NC and NC/DPA (2 mass%) were investigated at a heating rate of 0.3 K/min in dry air; the measured heat release temperatures of the samples (436 and 439 K, respectively) did not differ significantly. Moreover, the shapes of the thermal curves obtained for both these samples were very similar, as shown in Fig. 1a. In addition, the pressure behaviors of both NC and NC/DPA were nearly identical, as shown in Fig. 1b. As mentioned above, Nakamura et al. [14, 15] observed the thermal behaviors of NC and aged NC by DSC at a heating rate of 1–10 K/min and found that the heat release temperatures and the shapes of the thermal curves were almost identical for these samples. The thermal behavior of NC mixed with a destabilizer such as HNO3 was identical to that of pure NC. Our results were consistent with Nakamura’s results, i.e., there was no notable difference in the heat release temperatures of NC samples with different stabilities. Therefore, it could be speculated that a simple comparison of the thermal or pressure behaviors is not sufficient to evaluate the stability of NC unless NC is significantly destabilized in the presence of H2SO4 or water.
Activation energy
A comparison of the activation energies of pure NC and NC/DPA (2 mass%) revealed a characteristic difference between the two samples. The heating rate was varied from 0.02 to 0.3 K/min. Friedman’s formula (Eq. 1) was used to plot the value of ln(dx/dt) at the isoconversional point on each thermal curve as a function of 1/T, as shown in Fig. 2a–d. The slopes in each figure correspond to the activation energies of pure NC and NC/DPA.
Figure 2a shows the plots of ln(dx/dt) against 1/T at the isoconversional point 0.07; in this case, the activation energy of pure NC is lower than that of NC/DPA. The difference in the activation energies is particularly apparent at lower heating rates. Figure 2b shows the plots of ln(dx/dt) against 1/T at the isoconversional point 0.1; in this case, the plot for NC/DPA is linear, while that obtained for pure NC is not. This is because the plot for pure NC is inflected at 0.02 K/min. Figure 2c, d show the plots of ln(dx/dt) against 1/T beyond the isoconversional point 0.2; the plots for both pure NC and NC/DPA are linear, and their activation energies are approximately identical. By observing the activation energies of pure NC and NC/DPA at low heating rates at the beginning of the reaction, we can conclude that NC/DPA is more stable than pure NC.
Thermal behavior at low heating rates
The difference in the activation energies of pure NC and NC/DPA at the initial stages of the reaction at low heating rates is due to the small amount of heat released before the main heat release. As shown in Fig. 3, two exothermic peaks are observed at 0.02 K/min; however, the first peak diminishes at higher heating rates. The ratio of the heat released in the first peak to that released in the second stage increases with a decrease in the heating rate. This tendency is more apparent in the case of pure NC than in the case of NC/DPA. This implies the difference in the activation energies of pure NC and NC/DPA, as shown in Fig. 2a, results from the reaction amount of the first peak.
By studying the thermal curves at 0.02 K/min (Fig. 3), we speculate the reactions that correspond to each of the two exothermic peaks. In an earlier study [4–6], we investigated the heat release mechanism in NC and found that the amount of heat released in the first step depends on the presence of atmospheric O2. As shown in Fig. 4, the amount of heat corresponding to the first peak increases with the partial pressure of O2 in the atmosphere, and this behavior is not observed in a N2 atmosphere. From these results, we concluded that the first heat release results from oxidation of NC by atmospheric O2. We also concluded that the second heat release is the result of a reaction with intramolecular O2 because this heat release is observed in a N2 atmosphere as well. No difference in the thermal behaviors of pure NC and NC/DPA could be detected in the thermal analyses conducted at high heating rates, probably because the decomposition of NC itself (attributed to the second peak) occurs before the oxidation of NC by atmospheric O2, which corresponds to the first peak. Hence, similar exothermic peaks were obtained in the thermal curves of pure NC and NC/DPA at high heating rates.
Effect of storage atmosphere on the thermal curve of pure NC [4]
Next, we study the thermal curves in order to evaluate the stability of NC. The stability of NC depends on the first heat release because its amount was influenced by the initial amount of stabilizer that means the stability of NC. In considering the use as a stability evaluation method, however, the difference between pure NC and NC/DPA is also small. In addition, the pressure curves of pure NC and NC/DPA exhibit nearly identical behavior. In this study, we assume that when measurements are carried out at a high O2 partial pressure under isothermal conditions, the amount of heat released in the first step will increase, and differences in the thermal and pressure behaviors of pure NC and NC/DPA will be apparent.
Thermal behavior under isothermal conditions
On the basis of the abovementioned assumption, we carried out measurements in pure O2 atmosphere under isothermal conditions. The storage temperature was 393 K; this temperature is lower than the decomposition temperature of NC. The results are shown in Fig. 5a. The heat released corresponding to the first peak increased for both NC and NC/DPA, and the induction period of the heat release for NC/DPA (2 mass%) was significantly longer than that for pure NC. The induction period is defined as the intersection of the tangent at the maximum inclination point of the heat flow and the baseline. The induction periods for pure NC and NC/DPA were 7 and 27 h, respectively. As seen from Fig. 5b, the pressure behaviors of pure NC and NC/DPA were similar to their corresponding thermal behaviors. The induction period of the pressure change for NC/DPA was longer than that for pure NC. The relatively stability of NC could be determined from the abovementioned measurements carried out in an O2 atmosphere under isothermal conditions.
Comparison of the results of the thermal analyses with those of ABEL heat test and the effect of the type of stabilizer on the stability of NC
We compared the results of our experiments with those of the ABEL heat test, which the mandatory stability test in Japan. The elongation ratio for the induction period of the first heat release of NC/DPA was correlated with the results of the ABEL heat test. Table 1 lists the values of the induction periods determined from the ABEL heat test conducted in an earlier study [13] and the induction periods of the first heat release for pure NC, NC/DPA (2 mass%), and NC/DPA (3 mass%). From these results, the induction of heat was related to that of the ABEL test.
We investigate the thermal behaviors of NC and NC/AKII (2 mass%) (AKII is a conventional NC stabilizer) under isothermal conditions at 393 K in an O2 atmosphere. The induction period of NC/AKII is longer than that of pure NC, as shown in Fig. 6. These results indicate that the relative stability of NC can be determined by carrying out measurements under the abovementioned conditions. The results obtained from the ABEL heat test are in good agreement with those obtained in our experiments performed using pure NC and NC/AKII; these results are also listed in Table 1. Kimura et al. suggested that volatility of the stabilizer is conducive to the results of ABEL heat test [16]. From the abovementioned suggestion, we speculate that the stability of NC/AKII has been underestimated. This is because AKII, which has a lower volatility than DPA, is less reactive toward NO2 in the gas phase than DPA. Therefore, the stability of NC/AKII needs to be evaluated comprehensively by the ABEL heat test as well as by thermal analyses.
Conclusions
We investigated the effect of heating rate on the thermal behaviors of NC samples with different stabilities for the following reasons: (1) to identify why temperature thermal analyses under cannot be used to evaluate the stability of NC and (2) to identify the conditions under which thermal analyses can be used to estimate the stability of NC. From our experiments, we could draw the following conclusions:
-
The thermal behaviors of pure NC and NC/DPA were observed at a heating rate of 0.3 K/min in dry air; the measured heat release temperatures were approximately same for these samples. Moreover, the shapes of the thermal curves and the pressure behaviors obtained for NC and NC/DPA were also practically similar. Therefore, we concluded that the stability of NC cannot be evaluated by simply comparing the thermal or pressure behaviors unless NC is significantly destabilized in the presence of excess H2SO4.
-
At low heating rates (approximately 0.02 K/min), two exothermic peaks were observed in the thermal curves of NC and NC/DPA. The ratio of the heat released in the first step to that released in the second step increased with a decrease in the heating rate. This tendency was more apparent in the case of pure NC than in the case of NC/DPA. This indicated that the stability of NC depended on the first heat release because its amount was changed by the amount of stabilizer that means the stability of NC. Therefore, we concluded that the stability of NC could be evaluated by studying the first heat release.
-
At a high heating rate, no difference could be detected in the thermal behaviors of pure NC and NC/DPA, probably because of the decomposition of NC itself (attributed to the second peak) occurs before the oxidation of NC by atmospheric O2, which corresponds to the first peak. Hence, similar exothermic peaks were obtained in the thermal curves of pure NC and NC/DPA at high heating rates.
-
The measurement was performed in pure O2 atmosphere under isothermal conditions. The storage temperature was 393 K. The heat released in the first step increased for both pure NC and NC/DPA, and the corresponding induction period of NC/DPA was significantly longer than that of pure NC. In addition, the induction period for pressure change for NC/DPA was longer than that for pure NC. These measurements carried out in an O2 atmosphere under isothermal conditions are expected to be useful in determining the relative stability of NC.
References
Editorial Department of Japan Explosives Society, Ippan Kayakugaku, Japan Explosives Society; 1998. p. 172 (in Japanese).
Osada H. Kayaku Chemistry. Maruzen 2003;190–192 (in Japanese).
URL of Relational Information System for Chemical Accidents Database, http://riodb.ibase.aist.go.jp/riscad/PHP_EN/index.php.
Katoh K, Le L, Itoh M, Arai M, Tamura M. Study on the spontaneous ignition of cellulose nitrate: effect of the type of storage atmosphere (I). Sci Tech Energ Mater. 2003;64:236–40.
Katoh K, Le L, Itoh M, Arai M, Tamura M. Study on the spontaneous ignition of cellulose nitrate: effect of the type of storage atmosphere (II). Sci Tech Energ Mater. 2004;65:77–81.
Katoh K, Le L, Kumasaki M, Wada Y, Arai M, Tamura M. Study on the spontaneous ignition mechanism of nitric esters (I). Thermochim Acta. 2005;431:161–7.
Lussier LS, Gagnon H, Bohn MA. On the chemical reactions of diphenylamine and its derivatives with nitrogen dioxide at normal storage temperature conditions. Propellant Explos Pyrotech. 2000;25:117–25.
Lussier LS, Bergeron E, Gagnon H. Study of the daughter products of Akardite-II. Propellant Explos Pyrotech. 2006;31:253–62.
Brownlie IT, Ingold KU. The inhibited autoxidation of styrene: part V. The kinetics and deuterium isotope effect for inhibition by diphenylamine, phenyl-alpha-naphthylamine, and phenyl-beta-naphthylamine. Can J Chem. 1966;44:861–8.
Nishimura N, Nakamura T, Sueishi Y, Yamamoto S. Spin trapping as applied to radicals produced by chemical reaction. III. Intermediates of the reactions of dibenzoyl peroxide with diethylamine and diphenylamine. Bull Chem Soc Jpn. 1994;67:165–71.
JIS K4822, Reagents for stability test of explosives.
Nakamura J, Nakamura H, Hika K, Kanezawa T, Hirosaki Y, Torikai T, Kitajima E, Nishi S. Proceedings of the annual conference of Japan Explosives Society. Tokyo, Japan; 2002. p. 65.
Katoh K, Nakahama M, Wada Y, Ogata Y, Arai M. Proceedings of the annual conference of Japan Explosives Society. Kitakyushu, Japan; 2007. p. 53.
Nakamura H, Nishi M, Akiyoshi M, Hara Y. Thermal degradation of nitrocellulose at low temperature. J Jpn Explos Soc. 2002;63:121–7.
Nakamura H, Matsuura N, Akiyoshi M, Hara Y. The exothermal decomposition of nitrocellulose in mixed acids. J Jpn Explos Soc. 2000;61:108–13.
Kimura Jun-ichi. Chemical sensor applied stability test of energetic materials. Explosion. 2000;10:2–10.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Katoh, K., Ito, S., Kawaguchi, S. et al. Effect of heating rate on the thermal behavior of nitrocellulose. J Therm Anal Calorim 100, 303–308 (2010). https://doi.org/10.1007/s10973-009-0478-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-009-0478-5