Abstract
To investigate the molecular interaction of amyloid beta peptides Aβ1–28 or Aβ25–40 with model lipid membranes differential scanning calorimetry (DSC) and DPH and TMA DPH fluorescence anisotropy approaches were used. The main transition temperature (T m) and enthalpy change (ΔH) of model lipid membranes composed of DMPC/DPPG on addition of Aβ25–40 or Aβ25–40 at 10:1 (w/w) phospholipid/peptide ratio either non-aggregated or previously aggregated were examined. The effect of Aβ1–28 and Aβ25–40 on the membrane fluidity of liposomes made of DMPC/DPPG (98:2 w/w) was determined by fluorescence anisotropy of incorporated DPH and TMA DPH. The results of this study provide information that Aβ1–28 preferentially interacts with the hydrophilic part of the model membranes, while Aβ25–40 rather locates itself in the hydrophobic core of the bilayer where it reduces the order of the phospholipids packing.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ordered protein aggregation with amyloid fibril formation and its accumulation in body tissues particular in brain lead to the range of the severe neurodegenerative diseases like Alzheimer’s (AD), Parkinson’s and other diseases [1–4]. In these pathological situations, structural changes affecting proteins otherwise normally expressed in the organism lead to the formation of proteinase resistant fibrils composed of the aggregated abnormal forms of the proteins. These aggregates are rich in β-sheet structure, and they are the main component of the amyloid plaques in the affected central nervous systems [5–8]. The mechanism by which Aβ damages cells is still under debate [9, 10] but the hypotheses that non-specific interaction between Aβ proteins and neuronal membranes plays an important role in the conformational changes, degradation and aggregation of these peptides were discussed in literature [11–14]. A number of observations indicate that the primary target of amyloid and prion peptides is the cell membrane of neurons [12, 15, 16]. It was previously reported that interactions with lipid membranes, can alter important biological properties of Aβ proteins [13, 17–20]. Moreover, the presence of negatively charged lipids induces conformational changes of Aβ native form to β-sheet structures [21]. Model lipid membranes composed of synthetic phospholipids have been extensively used to investigate the amyloid peptide interaction with membranes [15, 22–24].
In this paper, we have analyzed the effects of two amyloid beta peptides (Aβ1–28 and Aβ25–40) on the thermotropic properties of the model lipid membranes using DSC and on the fluidity of liposomes composed of DMPC/DPPG by analysis of the fluorescence anisotropy of DPH and TMA DPH incorporated in the liposomes. The amino acid sequence of the Aβ25–40 peptide fragment provides hydrophobic groups which enable this peptide to be incorporated into the hydrophobic part of lipid membranes.
Results also show that the investigated peptides affect the bilayer order of DMPC membrane in the temperature range of the cooperative phase transition. The incorporation of Aβ25–40 in to the DMPC/DPPG vesicles decreases the bilayer fluidity.
It was shown that the effect of Aβ25–40 peptide incorporation into the lipid membranes caused bigger alterations in membrane properties than Aβ1–28 peptide fragment.
Experimental
Materials
Synthetic peptides Aβ1–28 [DAEFRHDSGYEVHHQKLVFFAEDVGSNK] and Aβ25–40 [SNKGAIIGLMVGGVV] were purchased from JPT Peptide Technologies (Germany). Stock solutions were kept in aqueous 10 mM Hepes buffer at neutral pH. Phospholipids (DMPC, DPPG) and fluorescent probes (DPH, TMA DPH), were purchased from Sigma Chemical Company.
Differential scanning calorimetry
DSC was performed on a DSC 822e calorimeter (Mettler Toledo, Switzerland) calibrated using pure indium (T m = 156.6 °C). The samples were prepared by adding appropriate amount of DMPC/DPPG mixtures (98:2 w/w) dissolved in chloroform and dried under vacuum. 2-3 mg of dried residue was accurately weighed in aluminum crucibles of 40 μL capacity. 10% (w/w) of Aβ1–28 or Aβ25–40 was added to the samples followed by hydration with HPLC grade water filtered through Millipore Filters (pore size 200 nm) in a ratio of sample/H2O, 1:2 (w/v). The peptide aggregation process was triggered by the addition of heparin and the crucibles were hermetically sealed. The same experiment was repeated using previously aggregated peptides.
Prior to DSC scanning, the samples underwent a quick heating and a quick cooling cycle (scanning rate of 10 and 20 °C/min, respectively) to ensure equilibration and exemption of the thermal history of the samples. All samples were scanned three to four times from 0 to 40 °C until identical curves were obtained using a scanning rate of 1 °C/min. An empty pan was used as a reference. Enthalpies and characteristic temperatures were calculated using Mettler-Toledo STAR software [25].
Liposome preparations
Large unilamellar vesicles (LUV) composed of DMPC/DPPG (98:2 w/w) were prepared using the thin-film hydration method. Briefly, appropriate amounts of lipid solutions in chloroform were placed in a round bottom flask and the thin lipidic film was formed by slow removal of the solvent under argon atmosphere. The remaining solvent traces were removed under vacuum using a rotary evaporator over a water bath at 37 °C for 30 min. The resulting lipid film on the wall of the flask was hydrated with an appropriate volume of buffer resulting in a final lipid concentration of 5 mg/mL. The mixture was vortexed for 5 min with glass beads, allowed to equilibrate for 30 min, under argon atmosphere at 37 °C (above the gel–liquid crystal transition temperature of the lipid mixture). Subsequently the liposome suspension was forced to pass at least 15 times through a polycarbonate membrane of 100 nm porosity (Nuclepore, T-E), mounted in a mini-extruder (Avanti Polar Lipids) fitted with two 1,000-μL Hamilton gastight syringes. Exposure to light was minimized throughout the liposome preparation process. The size distribution (z-average mean), polydispersity index of the liposomes were measured at room temperature using dynamic light scattering in a photon correlation spectrometer (Zetasizer Nano-S90, Malvern Instruments, Malvern UK). The refraction factor was assumed 1.336 while the detection angle was 90” and the wavelength was 633 nm. The analysis method used was based on CONTIN algorithm.
Membrane fluidity measurements
The fluidity of lipid bilayer was measured using a steady-state fluorescence polarization technique. Two different fluorescent probes were used: 1,6-diphenyl-1,3,5-hexatriene (DPH), an apolar molecule which is incorporated into the hydrophobic region of the liposome bilayer with its long axis parallel to the acyl chains, and 1-(4-trimethylammoniumphenyl)-6-phenyl-1,3,5-hexatriene (TMA-DPH), which is anchored at the surface of the liposome bilayer in contact with the water due to its positively charged amino groups. Since DPH probe is incorporated deeper into the lipid bilayer than TMA-DPH, the use of both probes in the same lipid membrane allows for the comparison of membrane order at different depths of the bilayer. Measurements were made using a Perkin Elmer luminescence spectrometer LS-50B equipped for fluorescence polarization measurement. A quantity of 500 μM liposomes suspension was added to the cuvette followed by the addition of 1 μM DPH (in tetrahydrofuran) or TMA-DPH (in methanol) molar ratio 1:500 (probe/phospholipids). The sample was stirred well and incubated in the dark at room temperature for 20 min. The cuvette holder was temperature controlled by water thermostat (MLW-U) with 37 °C. The readings were taken at intervals of 2 s. The polarization values (r) of the samples were calculated by the fluorescence data manager program using the following equation:
where I VV and I VH are the vertical and horizontal fluorescence intensities, respectively, to the vertical polarization of the excitation light beam. The factor G = I HV/I HH (grating correction factor) corrects the polarizing effects of the monochromator. The excitation wavelength was 348 nm and the fluorescence emission was measured at 426 nm for DPH and 340 nm; 430 nm for TMA DPH, respectively.
Results
DSC experiments
Differential scanning calorimetry profiles for model lipid membranes composed of DMPC/DPPG 98:2 (w/w) either pure or in the presence of 10% (by weight) of Aβ1–28 or Aβ25–40 are shown in Fig. 1 and the corresponding thermodynamic parameters are reported in Table 1. The DMPC/DPPG membrane curve shown in Fig. 1a shows two characteristic peaks at 14.26 and 24.54 °C. The first peak presents a low enthalpy transition attributed to the mobility of the choline polar head of DMPC, while the sharp enthalpy main transition is attributed to the mobility of the alkyl chains [25–28]. The presence of both Aβ1–28 and Aβ25–40 peptide fragments in the DMPC/DPPG lipid membrane caused the increase of the transition temperature (T m) and transition enthalpy (ΔH) of the pre-transition peak (Table 1). Thermal analysis of the main peak shows that Aβ1–28 peptide fragment induces a minor change of T m from 24.54 to 24.67 °C. In contrast, the presence of Aβ25–40 peptide in the membrane leads to the increase of T m from 24.54 to 25.11 °C and of enthalpy ΔH from 28.28 to 36.59 kJ/mol−1 (Table 1). These data lead to the conclusion that Aβ1–28 peptide fragment interacts with the external surface of the membrane (DPPC/DPPG + Aβ1–28 systems), while Aβ25–40 is rather located in the hydrophobic core of the bilayer were it reduces the order of the phospholipids packing. This can be explained by considering the different nature of investigated peptide fragments. Therefore, two different mechanisms can be distinguished. In the case of Aβ1–28 the interactions with a lipid bilayer are driven by electrostatic forces. On the contrary, for Aβ25–40 a predominant role is played by hydrophobic interactions.
We also carried out experiments on multilamellar DMPC/DPPG membranes interaction with previously aggregated peptides to examine the possibility of peptides in a plaque form to change the thermodynamic parameters of model membranes. Samples with aggregated peptides were prepared before DSC measurements by the addition of corresponding amounts of heparin to the dissolved peptides. It should be noted that pure heparin had no effect on the thermodynamic parameters of DMPC/DPPG lipid system (data not shown).
The DSC scans for the DPPC/DPPG with Aβ1–28 or Aβ25–40 aggregates are shown in Fig. 2. The presence of Aβ1–28 in the DPPC/DPPG lipid membranes induces the small increase of the pre-transition and main peaks temperatures from 14.26 to 15.36 °C and 24.54 to 24.70 °C, respectively. Transition enthalpy of pre-transition peak changes from 3.66 to 3.76 kJ/mol−1 and for main peak from 28.28 to 26.83 kJ/mol−1. The presence of Aβ25–40 in the same lipid system induces following changes of thermodynamical parameters of melting process: temperatures of pre-transition and main peaks rice from 14.26 to 14.68 °C and from 24.54 to 24.76 °C, respectively. Transition enthalpies of pre-transition and main peaks change from 3.66 to 3.96 kJ/mol−1 and from 28.28 to 25.77 kJ/mol−1, respectively (Table 2). These data suggest that changes of heating curves in the presence of aggregated peptides were insignificant leading to the conclusion that peptides’ plaques show weak interaction with lipid membranes and do not change the order of the phospholipids packing in the bilayer.
These results are consistent with literature findings. Recent studies have shown that plaques formed from aggregated peptides are not as harmful as short oligomers [29]. In terms of interactions with a lipid bilayer, this phenomenon can be easily explained. The efficient incorporation into a lipid bilayer can happen only when the object does not exceed certain dimensions. It seems that the size of aggregated peptides that form fibrils is too big to allow for the incorporation.
Fluorescent anisotropy experiments
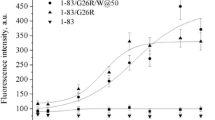
To confirm the effects of Aβ1–28 or Aβ25–40 on model membranes obtained by the DSC method we also carried out experiments of membrane fluidity changes determination of LUV (liposome diameter was measured to be equal to 162.9 nm) induced by incorporation of the investigated peptide fragments and measured by DPH and TMA DPH fluorescence anisotropy. DPH shows a stiff structure and possesses cylindrical symmetry around its long axis in the saturated lipid system [30]. DPH is likely aligned with the long axis parallel to lipid acyl chains [31]. The hydrophilic group of the TMA DPH anchors the molecule in the head group region of the bilayer [32]. The effect of Aβ1–28 and Aβ25–40 on the membrane fluidity of LUVs made of DMPC/DPPG (98:2 w/w) and determined by fluorescence anisotropy of incorporated DPH and TMA DPH is shown in Fig. 3. The addition of progressive concentrations of peptides leads to a change of probe anisotropy that shows that the membrane fluidity alters. For Aβ1–28 we observed significantly rising anisotropy of TMA DPH but not DPH. The increase of TMA DPH anisotropy was dose-dependent in the concentration range from 10 to 50 μM. It may mean that this peptide fragment interacts with the polar region of the membrane. Aβ25–40 increased fluorescence anisotropy of DPH and slightly of TMA DPH. It can be considered that this peptide fragment interacts with a hydrophobic part of membrane and slightly with a hydrophilic region. This indicates that both peptides change the fluidity of the liposome lipid matrix. This change was significantly pronounced at higher concentrations of the preparations studied. Comparison of the changes in anisotropy with increasing concentration showed that the two peptides had completely different effects. It should be noted that Aβ25–40 is insoluble in water, so it was diluted in DMSO. Contribution of DMSO was subtracted from each measurement point for Aβ25–40, so the changes in TMA DPH and DPH anisotropy are attributable only to the interaction of peptide with lipid molecules in the bilayer. It may be considered that the sample molecules interact directly with the lipid. This result is highly consistent with the DSC data.
Fluorescent anisotropy of TMA DPH and DPH in phospholipids/peptide mixtures. Liposomes were prepared using DMPC/DPPG (98:2) w/w phospholipids. (0.5 mM of lipid, 10 mM HEPES buffer, pH 7.4, 37 °C). Lipid:probe (500:1) molar ratio. Final peptide concentration was 50 μM. L:P (10:1) final molar ratio. (Circle), Aβ1–28; (square), Aβ25–40. a TMA DPH anisotropy detected with 1 μM of probe. This method estimates membrane fluidity in the membrane interface region; b DPH anisotropy detected with 1 μM of probe. This method estimates membrane fluidity in the hydrophobic membrane interior region
Discussion
Our results show that Aβ interact with DMPC/DPPG (98:2) w/w model lipid membranes. Differential scanning calorimetry demonstrated that amyloid peptide fragments Aβ1–28 and Aβ25–40 altered the thermotropic behavior of DMPC/DPPG model membranes. Aβ1–28 caused an increase in the thermal phase transition temperature of pre-transition peak and its transition enthalpy without affecting the thermal parameters of the main transition. Aβ25–40 affected the transition temperature and enthalpy of both pre-transition and main peaks of DMPC/DPPG membranes (Fig. 1b, c). These DSC results suggest that Aβ1–28 inserts into the headgroup region of the liquid-crystalline DMPC/DPPG. Aβ25–40 had a much greater effect on membrane and inserts deeper into the bilayer of DMPC/DPPG resulting in an increase in the area per lipid molecule and leading to a decrease of the melting cooperativity of the main phase transition. The amino acid sequence of the Aβ25–40 peptide fragment provides hydrophobic groups which renders this peptide capable of being incorporated into the hydrophobic part of lipid membranes. The proposed localization of Aβ1–28 and Aβ25–40 in the DMPC/DPPC bilayer is presented in Fig. 4. Aggregated peptides, behaved in a qualitatively different manner. In both cases no significant effects on the lipid bilayer were observed, however, aggregated fragments caused only a small decrease in enthalpy of phase transition (Table 2). These data imply that aggregated peptides interact probably with membranes in a manner of random contact but this supposition needs further confirmation by other methods.
As the influence of amyloid peptides on membrane order is concerned, the results concerning DPH and TMA DPH fluorescence anisotropy indicate that Aβ1–28 and Aβ25–40 perturb the packing order of the bilayer. The progressive increase in TMA DPH fluorescence anisotropy with increasing concentration of Aβ1–28 in the bilayer can be explained by interaction of this fragment with the hydrophilic part of the membrane and partial incorporation into the hydrophobic region which is reflected by the small increase in the anisotropy of DPH fluorescence in liposomes containing Aβ1–28. This suggests that the Aβ1–28 in the bilayer affects both the lipid-water interphase and hydrophobic core of the membrane. In contrast, the effect of Aβ25–40 on anisotropy of DPH fluorescence was more pronounced. Thus, it is possibly due to the interaction of Aβ25–40 with the acyl chains of the lipids and leads to the conclusion of the Aβ25–40 incorporation into the hydrophobic part of the bilayer.
Summarising, Aβ1–28 probably interacts with polar heads of lipids but Aβ25–40 resides within the hydrocarbon core in model membranes. This localization may be important in determining its capacity to interact with membrane lipids.
Conclusions
This study provides the direct experimental evidence for Aβ1–28 and Aβ25–40 peptide fragments’ interactions with the DMPC/DPPG lipid bilayer. Results show that Aβ25–40 can incorporate deeper into the lipid bilayer than Aβ1–28. The above observation supports the strong interaction of Aβ25–40 in lipid/peptide ratio (10:1) w/w with the DMPC/DPPG membranes. On the other hand Aβ1–28 peptide fragment probably interacts only with a hydrophilic part of a lipid bilayer.
These results complement studies, which have concluded that Aβ1–28 and Aβ25–40 peptides’ interaction with lipid membranes governed by their ability to change the membrane lipids packing orders [9, 14, 15]. Finally these data may have important implications for membrane structure/function relationship in Alzheimer’s disease neuropathology. All this findings can have important consequences on the understanding of the mechanism of interaction of amyloid peptides with cells.
Abbreviations
- Aβ:
-
β-amyloid peptide
- AD:
-
Alzheimer’s disease
- Buffer:
-
10 mM Hepes buffer pH-7.4
- Cp:
-
Heat capacity
- DMPC:
-
1,2-dimyristoyl-sn-glycero-3-phosphocholine
- DPPG:
-
Dipalmitoylphosphatidylglycerol
- DPH:
-
1,6-diphenyl-1,3,5-hexatriene
- DSC:
-
Differential scanning calorimetry
- ΔH :
-
Main phase-transition enthalpy
- L:P:
-
Lipid:peptide ratio
- LUV:
-
Large unilamellar vesicles
- T m :
-
Gel to liquid-crystalline phase transition temperature
- TMA-DPH:
-
1-[4-(trimethyl-ammonium) phenyl]- 6-phenyl-1,3,5-hexatriene
References
Prusiner SB. Prions. Proc Natl Acad Sci USA. 1998;95:13363–83.
Dobson CM. Protein folding and misfolding. Nature. 2003;426:884–90.
Revesz T, Ghiso J, Lashley T, Plant G, Rostagno A, Frangione B, et al. Cerebral amyloid angiopathies: a pathologic, biochemical, and genetic view. J Neuropathol Exp Neurol. 2003;62:885–98.
Temussi PA, Masino L, Pastore A. From Alzheimer to Huntington: why is a structural understanding so difficult?. EMBO J. 2003;22:355–61.
Serpell LC. Alzheimer’s amyloid fibrils: structure and assembly. Biochim Biophys Acta. 2000;1502:16–30.
Rottkamp CA, Atwood CS, Joseph JA, Nunomura A, Perry G, Smith MA. The state versus amyloid-beta: the trial of the most wanted criminal in Alzheimer disease. Peptides. 2002;23:1333–41.
DeMarco ML, Daggett V. From conversion to aggregation: protofibril formation of the prion protein. Proc Natl Acad Sci USA. 2004;101:2293–98.
Klajnert B, Cladera J, Bryszewska M. Molecular interactions of dendrimers with amyloid peptides: pH dependence. Biomacromolecules 2006;7:2186–91.
Sabate R, Gallardo M, Estelrich J. Spontaneous incorporation in β-amyloid peptide into neutral liposomes. Colloids Surf. 2005;270(271):13–7.
Behbehani GR, Mirzaie M. A high performance method for thermodynamic study on the binding of copper ion and glycine with Alzheimer’s amyloid β peptide. J Therm Anal Calorim. 2009;96:631–5.
Pillot T, Goethals M, Vanloo B, Talussot C, Brasseur R, Vandekerckhove J, et al. Fusogenic properties of the C-terminal domain of the Alzheimer b-amyloid peptide. J Biol Chem. 1996;271:28757–65.
Choo-Smith LP, Garzon-Rodriguez W, Glabe CG, Surewicz WK. Acceleration of amyloid fibril formation by specific binding of Ab-(1–40) peptide to ganglioside-containing membrane vesicles. J Biol Chem. 1997;272:22987–90.
Barnham KJ, Masters CL, Bush AI. Neurodegenerative diseases and oxidative stress. Nat Rev Drug Discov. 2004;3:205–14.
Kaufer JN, Sorrentino J, Sitar D. Amyloid beta peptide membrane perturbation is the basis for its biological effects. Neurochem Res. 1999;24:1621–30.
Ambroggio EE, Kim DH, Separovic F, Barrow CJ, Barnham KJ, Bagatolli LA, et al. Surface behavior and lipid interaction of Alzheimer β-amyloid peptide 1–42: a membrane-disrupting peptide. Biophys J. 2005;88:2706–13.
Abramov AY, Canevari L, Duchen MR. Beta-amyloid peptides induce mitochondrial dysfunction and oxidative stress in astrocytes and death of neurons through activation of NADPH oxidase. J Neurosci. 2004;24(2):565–75.
Murray IVJ, Sindoni ME, Axelsen PH. Promotion of oxidative lipid membrane damage by amyloid β proteins. Biochemistry. 2005;44(37):12606–13.
Kevin J, Roberto C, Konrad B, Colin L, Hill M. Delineating common molecular mechanisms in Alzheimer's and prion diseases. Trends Biochem Sci. 2006;31(8):465–72.
Mason RP, Trumbore MW, Pettegrew JW. Membrane interactions of a phosphomonoester elevated early in Alzheimer’s disease. Neurobiol Aging. 1995;16(4):531–9.
Abramov AY, Canevari L, Duchen MR. Calcium signals induced by amyloid β peptide and their consequences in neurons and astrocytes in culture. Biochim Biophys Acta. 2004;1742:81–7.
Terzi E, Holzemann G, Seeling J. Self-association of β-amyloid peptide (1–40) in solution and binding to lipid membranes. J Mol Biol. 1995;252:633–42.
Kremer JJ, Sklansky DJ, Murphy RM. Profile of changes in lipid bilayer structure caused by β-amyloid peptide. Biochemistry 2001;40(29):8563–71.
Alarco′n JM, Brito JA, Hermosilla T, Atwater I, Mears D, Rojas E. Ion channel formation by Alzheimer’s disease amyloid b-peptide (Ab40) in unilamellar liposomes is determined by anionic phospholipids. Peptides. 2006;27:95–104.
Grasso D, Milardi D, La Rosa C, Rizzarelli E. DSC study of the interaction of the prion peptide PrP 106-126 with artifical membranes. New J Chem. 2001;25:1543–48.
Gardikis K, Hatziantoniou S, Viras K, Demetzos C. Effect of a bioactive curcumin derivative on DPPC membrane: a DSC and Raman spectroscopy study. Thermochim Acta. 2006;447:1–4.
Antunes-Madeira MC, Videira RA, Madeira VMC. Effects of parathion on membrane organization and its implications for the mechanisms of toxicity. Biochim Biophys Acta. 1994;1190:149–54.
Gardikis K, Hatziantoniou S, Viras K, Wagner M, Demetzos C. A DSC and Raman spectroscopy study on the effect of PAMAM dendrimer on DPPC model lipid membranes. Int J Pharm. 2006;318:118–23.
Pentak D, Sulkowskil WW, Sulkowska A. Calorimetric and EPR studies of the thermotropic phase behavior of phospholipid membranes. J Therm Anal Calorim. 2008;93:471–7.
Benseny-Cases N, Cocera M, Cladera J. Conversion of non-fibrillar beta-sheet oligomers into amyloid fibrils in Alzheimer's disease amyloid peptide aggregation. Biochem Biophys Res Commun. 2007;361:916–21.
Wang S, Beghem JP, Gradton E, Glasser M. Orientational distribution of 1,6-diphenyl-1,3,5-hexatriene in phospholipid vesicles as determined by global analysis of frequency domain fluorimetry data. Biochemistry. 1991;30:5565–72.
Zannoni C, Arcioni A, Cavatora P. Fluorescence depolarisation in liquid crystals and membrane bilayers. Chem Phys Lipids. 1983;32:179–250.
Moya-Quiles MR, Munoz DE, Vidal CJ. The pyrethroid insecticide deltamethrin modifies the thermotropic properties and lipid packing order of model membranes. Chem Phys Lipids. 1996;83:61–9.
Acknowledgements
This work was supported by POL-POSTDOC III (PBZ/MniSW/07/2006/22) Nr D077/P01/2007, Ministry of Science and High education, Poland.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ionov, M., Klajnert, B., Gardikis, K. et al. Effect of amyloid beta peptides Aβ1–28 and Aβ25–40 on model lipid membranes. J Therm Anal Calorim 99, 741–747 (2010). https://doi.org/10.1007/s10973-009-0405-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-009-0405-9