Abstract
Some metal complexes of norfloxacin (NOR) with the formula [M(NOR)2]X2·nH2O [M = Zn(II), (X = Cl−, AcO−, Br− and I−), Cd(II), (X = Cl−, NO3 − and SO4 2−) and Hg(II) (X = Cl−, NO3 − and AcO−)] have been synthesised and studied using elemental analysis (CHN), electronic (UV–vis, mid infrared, mass, and 1H-NMR spectra), TG and DTA. The thermal decomposition processes of these complexes were discussed. The Correlation coefficient, the activation energies, E*, the pre-exponential factor, A, the entropies, S*, enthalpies, H* and Gibbs free energies, G*, of the thermal decomposition reactions have been derived from thermogravimetric (TG) and differential thermogravimetric (DTG) curves. The characterization of the final products of the decomposition was achieved by IR spectra and X-ray powder diffraction (XRD). Using the Coats–Redfern and Horowitz–Metzeger methods, kinetic analysis of the thermogravimetric data is performed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
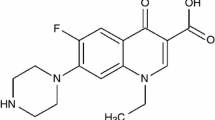
Norfloxacin (NOR; Fig. 1), 1-ethyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-3-quinolinecarboxylic acid, belongs to the family of molecules known as the fluoroquinolones and is a wide-ranging drug used in treating bacterial infections of the urinary tract, the respiratory tract, and the skin, amongst others. It is also known that NOR can be effective in treating diarrhoea, and could in addition treat conjunctivitis when it was administered in the form of eye drops. Norfloxacin was not, however, effective against infections involving anaerobic bacteria (e.g. yeast, athlete’s foot) [1–6].
When dealing with the interaction between drugs and metal ions in living systems, a particular attention has been paid to the interaction of metal ions with antibiotics. Antibiotics that interact with metal ions constituted a class of drugs which has been widely used in medicine both for human beings and animals [1, 2]. In particular, the interaction between transition metals and β-lactamic antibiotics such as cephalexin had been recently investigated by several physicochemical and spectroscopic methods, and with detailed biological data [3–6].
Many drugs possess modified pharmacological and toxicological properties when administered in the form of metallic complexes. Probably the most widely studied cation in this respect is Cu(II), for which a host of low-molecular-weight Cu(II) complexes have been proved beneficial against several diseases such as tuberculosis, rheumatoid arthritis, gastric ulcers, and cancers [7–10]. There has been a tremendous growth in the study of drugs from quinolone family, which began with the discovery of nalidixic acid some over 40 years ago. Since then, the exponential growth of this family had produced more than ten thousand analogues [11].
The coordination chemistry of these drugs with metal ions of biological and pharmaceutical importance is of considerable interest. Norfloxacin is considered the best of the third generation quinolone family. There are several reports regarding the synthesis and crystal structure of metal complexes with quinolone derivatives [12–15].
Quinolone antibiotics could participate in the formation of complexes in a number of ways [16–20]. When in acidic media, quinolones are usually singly and/or doubly protonated making them unable to coordinate to the metal cations and, in such cases, only electrostatic interaction are observed between the drug and the metal ions [16–18]. On the other hand it was found that neutral quinolones in the zwitterionic state were capable of forming simple complexes (bidentate chelating) [19–21]. The quinolones could also act as bridging ligands and are, consequently, capable of forming polynuclear complexes [20, 21].
The synthesis and characterization of new metal complexes with quinolone antibacterial agents are of great importance for understanding the drug-metal ion interaction and taking into account their potential pharmacological use. The objective of this study is the isolation and characterization of the Zn(II), Cd(II) and Hg(II) complexes, as well as their characterization using spectroscopic and thermal analysis techniques. The thermal behaviour of these complexes was also studied. The antibacterial activity of the investigated complexes was tested against Escherichia coli (Gram −ve), Bacillus subtilis (Gram +ve) and antifungal activity was also investigated (tricoderma and penicillium activities).
Experimental
Chemicals
Norfloxacin used in the present study was obtained from the Egyptian International Pharmaceutical Industrial Company (EIPICO). All chemicals used for the preparation of the complexes were of analytical reagent grade, commercially available, used without further purification and received from different sources (Fluka and Aldrich).
Synthesis
Complexes with the general formula [M(NOR)2]X2·nH2O [M = Zn(II), (X = Cl−, CH3COO−, Br− and I−), Cd(II), (X = Cl−, NO3 − and SO4 2−) and Hg(II) (X = Cl−, NO3 − and CH3COO−)] were synthesized.
All the complexes were prepared as follows, employing a 1:2 (metal ions: NOR) ratio. A solution of 1.0 mmol of a salt of each Zn(II), Cd(II) or Hg(II) previously dissolved in 10 cm3 of distilled water was added to a solution of 1.0 mmol of norfloxacin in 50 cm3 of acetone. The resulting mixtures were heated at ~60 °C under reflux on a water bath for about 10 h and then cooled. The obtained complexes were separated from the reaction mixture by filtration, washed with boiling water and acetone and dried under vacuum over CaCl2.
Instruments
Elemental analysis was carried out by standard micro chemical methods using a Perkin-Elmer CHN 2400 and the metal contents were determined gravimetrically by ignition weighted samples in air atmosphere at 1,073 K to constant weight as the metal oxide forms. IR spectra were recorded on a Bruker II FT-IR spectrophotometer (KBr discs) in the range from 4,000 to 400 cm−1. 1H-NMR spectra were recorded on a Varian Gemini 200 MHz spectrometer using DMSO-d6 as solvent. Mass spectra were performed on an AEI MS 30 mass spectrometer at 70 eV. TG–DTG measurements were carried out under N2 atmosphere within the temperature range from room temperature to 1,073 K using a Shimadzu TGA-50H thermal analyzer. Electronic spectra were obtained using a Jenway 6405 Spectrophotometer with a 1 cm quartz cell. Molar conductivities in DMSO at 10−3 mol dm−3 concentration were measured on a Jenway 4010 conductivity meter. The X-ray powder diffraction patterns of the decomposition products of NOR complexes were recorded with a Rikagu diffractometer using Cu/Kα radiation.
The anion analysis was performed as follows: the complexes were dissolved in concentrated HNO3, and the obtained samples diluted with water to 25 cm3. The qualitative analysis of Cl− and SO4 2− ions were performed by reactions with AgNO3 and BaCl2 solutions, respectively.
Antibacterial investigation
The procedure described by Gupta et al. [22] as employed. The investigated isolates of bacteria were seeded in tubes with nutrient broth (NB). The seeded NB (1 cm3) was homogenized in the tubes with 9 cm3 of melted (318 K) nutrient agar (NA). The homogeneous suspensions were poured into Petri dishes. The holes (diameter 4 mm) were done in the cool medium. After cooling, 2 × 10−3 dm3 of the investigated compounds were applied using a micropipette. After incubation for 24 h in a thermostat at 298–300 K, the inhibition (sterile) zone diameters (including disc) were measured and expressed in mm. An inhibition zone diameter over 7 mm indicates that the tested compound is active against the bacteria under investigation.
The antibacterial activities of the investigated compounds were tested against Escherichia coli (Gram −ve), Bacillus subtilis (Gram +ve), as well as the antifungal activity (tricoderma and penicillium).
Results and discussion
The elemental analysis results are summarized in Table 1. These results, as well as the obtained mass spectra are in good agreement with the proposed formula.
The melting points of the complexes are higher than that of the free ligand, revealing that the complexes are much more stable than ligand. The molar conductance values of the complexes were found to be in the range from 30-to-60 Ω−1 cm2 mol−1 at 298 K, which indicates that the complexes are of a non-electrolytic nature [23]. The low conductivity values are in agreement with the low solubility of NOR complexes in water, ethanol, chloroform, acetone and most organic solvents. On the other hand, they are soluble in DMSO, DMF and concentrated acids.
IR data and bonding
The IR data to NOR and its complexes are listed in Table 2. The IR spectra of the complexes are compared with those of the free ligand in order to determine the coordination sites that may involved in chelation. There are some guide peaks, in the spectra of the ligand, which are useful in achieving this goal. The position and/or the intensities of these peaks are expected to be changed upon chelation. These guide peaks are listed in Table 2. The ν(OH), ν(C=O), ν asym(COO) and ν sym(COO) stretching vibrations are observed at 3,448, 1,727, 1,590 and 1,396 cm−1 for free NOR ligand. The participation of the carboxylate O atom in the complexes formation is evidenced from the shift in position of these bands to 3,276–3,427, 1,709–1,720 or the disappearance of the bands between 1,549–1,591 and 1,381–1,394 cm−1 for NOR–metal complexes. For comparison the carbonyl–O; ν(C=O), stretching vibration is found in the free ligand at 1,716 cm−1. This band is shifted to lower wavenumbers (1,621–1,632 cm−1) in the complexes indicating the participation of the carbonyl–O in coordination. New bands are found in the spectra of the complexes in the regions 524–555, 497–523 and 464–498, which are assigned to ν(M–O) stretching vibrations of coordinated water, carboxylate–O and carbonyl–O, respectively. Therefore, from the IR spectra, it is concluded that NOR behaves as neutral bidentate ligand and binds to the metal ions through protonated carboxylate O and carbonyl groups.
UV–vis spectra
The formation of the M(II) complexes was also confirmed by UV–vis spectra. The electronic absorption spectra of the ligand and its M(II) complexes in DMSO in the 200–600 nm range. It can be seen that free NOR has two distinct absorption bands. The first one at 285 nm may be attributed to π → π* transition of the heterocyclic moiety and benzene ring. The second band observed at 335 nm is attributed to n → π* electronic transition. In the spectra of the M(II) complexes, the two bands are hypochromically affected obviously, suggesting the ligand has changed to the zwitterionic form. The results clearly indicate that the ligand coordinate to metal(II) ions via carboxylic and ketone groups, which is in accordance with the results of the FT-IR spectra.
Mass spectra
In the mass spectra of [Zn(NOR)2]Cl2·2H2O, [Cd(NOR)2]NO3 and [Hg(NOR)2]NO3, intense mass peaks at m/z 319, 275, 233, 161, 107, and 56 are detected. The first mass peak corresponds to the [H-NOR]+ ion and the second one proceeds by loss of CO2 from the molecular ion at m/z 275 with intensity 72%, then the elimination of C2H4N leads to the formation of an ion at m/z = 233. In comparison between the NOR (ligand) and the three NOR complexes, the peak assigned to molecular ion m/z = 319 of NOR ligand is present in all three complexes, and new peaks appear at m/z = 65, 112 and 201 can be assigned to zinc(II), cadmium(II) and mercury(II) metal, respectively. These results are again consistent with the presence of direct metal-ligand bonding in the three NOR complexes.
1H-NMR spectra
The 1H NMR spectra further support the assignment of the coordination modes. Figure 2 shows the 1H-NMR spectrum of Zn(II) complex which was carried out in DMSO-d6 as a solvent. Upon comparison with the free ligand, the signal observed at 11 ppm can be assigned to the carboxylate OH. This signal disappears in the spectrum of the [Zn(NOR)2]Cl2·2H2O complex, which confirms the coordination of NOR ligand to the M(II) ions through the deprotonated carboxylic O group. Due to the different chemical environments, two signals are recorded for the quaternized nitrogen (–+NH2) at δ 2.50 and 2.77 ppm. The peak at δ 3.55 ppm can be assigned as coming from the water molecules of hydration, which were not detected in the spectrum of the free NOR ligand. The protons of the –CH2– group quartet have a total integral of two units with the values δ 4.50–4.80 ppm, while the –CH3 group (triplet) have an integral of three units with the values δ 1.40, 1.42 and 1.45 ppm.
Thermogravimetric analysis (TG)
In the present investigation, the heating rates were controlled at 283 K min−1 under nitrogen atmosphere and the weight loss was measured from ambient temperature up to ≅1,273 K. The data are listed in Table 3 and shown in Fig. 3. The weight losses for each chelate were calculated within the corresponding temperature ranges. The different thermodynamic parameters are listed in Table 4.
The thermogravimetric curve of [Cd(NOR)2]·Cl2·2H2O chelate shows three decomposition steps within the temperature range 303–1,273 K. The first steps of decomposition within the temperature range 303–413 K correspond to the loss of water molecules of hydration and Cl2 gas with a mass loss of 12.58% (calcd. 12.47%). The energy of activation was 37.98 kJ mol−1. The subsequent steps (413–1,273 K) correspond to the removal of the organic part of the ligand leaving metal oxide as a residue. The overall weight loss amounts to 86.34% (calcd. 85.03%). Meanwhile, the TG curve of the [Cd(NOR)2]·(NO3)2 chelate shows two stages of decomposition within the temperature range of 373–1,173 K. The first stage at 373–693 K corresponds to the loss of C16H18FN3O3 and NO3 molecules with mass loss of 44.63% (calcd. 43.60%). The energy of activation for this step was 71.66 kJ mol−1. The second step involves the loss of C16H18FN3O2 and NO3 molecules with a mass loss of 42.03% (calcd. 41.77%). The energy of activation for this step was 56.79 kJ mol−1. In addition, the [Cd(NOR)2]·SO4 complex decomposes in three successive steps within the temperature range 303–1,073 K with mass loss of 74.84% (calcd. 75.44%) leaving CdSO4 as residue. The activation energies were 96.38, 241.0 and 92.93 kJ mol−1 for the 1st, 2nd and 3rd steps, respectively (Table 4).
On the other hand, [Hg(NOR)2]Cl2 and [Hg(NOR)2](AcO)2 chelates exhibit three decomposition steps. The first step in the temperature range 323–423 and 303–373 K [mass loss = 7.81% (calcd. 7.80%) and 11.86% (calcd. 12.32%)] may accounted for the loss of Cl2 gas and C2H6 and 2CO2 gases for [Hg(NOR)2]Cl2 and [Hg(NOR)2](AcO)2 complexes, respectively. As shown in Table 3, the mass losses of the remaining decomposition steps amount to 68.86% (calcd. 68.46%) and 65.73% (calcd. 65.06%). They correspond to the removal of NOR molecules leaving HgO as a residue. The energy of activation for these steps was 59.40 and 49.82 and 61.89 and 67.83 kJ mol−1 for the 2nd and 3rd steps of [Hg(NOR)2]Cl2 and [Hg(NOR)2](AcO)2 complexes, respectively. The [Hg(NOR)2](NO3)2 complex was thermally decomposed in one decomposition step within the temperature range of 323–873 K. The estimated mass loss of 77.12% (calcd. mass loss = 77.56%) may be attributed to the liberation of the 2NO3 and 2 NOR molecules leaving HgO as a residue. The activation energy was 17.24 kJ mol−1.
The TG curves of the [Zn(NOR)2]Cl2·2H2O and [Zn(NOR)2](OA)2 chelates represent three decomposition steps as shown in Table 3. The first step of decomposition within the temperature range 313–403 and 303–423 K corresponds to the loss of hydrated water molecules and C2H3O2 with a mass loss of 4.71% (calcd. for 2H2O; 4.44%) and 7.02% (calcd. for C2H3O2; 7.18%) for [Zn(NOR)2]Cl2·2H2O and [Zn(NOR)2](OA)2 chelates, respectively. The energy of activation for this step was 46.75 and 112.7 kJ mol−1 for [Zn(NOR)2]Cl2·2H2O and [Zn(NOR)2](OA)2 chelates, respectively. The remaining steps of decomposition within the temperature range 403–1,173 and 423–1,273 K correspond to the removal of NOR ligands in the form of gases with an energy of activation for these steps of 113.6 and 65.91, and 47.81 and 70.13 kJ mol−1 for the 2nd and 3rd steps of [Zn(NOR)2]Cl2·2H2O and [Zn(NOR)2](OA)2 chelates, respectively. The overall weight losses amount to 90.57% (calcd. 90.02%) and 89.47% (calcd. 90.09%) for [Zn(NOR)2]Cl2·2H2O and [Zn(NOR)2](OA)2 chelates, respectively. The [Zn(NOR)2]Br2 complex was thermally decomposed in one decomposition step within the temperature range of 423–1,273 K. The estimated mass loss of 89.91% (calcd. mass loss = 90.65%) may be attributed to the liberation of the Br2 and 2(NOR) molecules leaving ZnO as a residue. The activation energy was 205.0 kJ mol−1. The starting and final products were confirmed using IR spectrometry and X-ray powder diffraction. As an example, the X-ray powder pattern of the final products of ZnO for the Zn(II) complexes is shown in Fig. 4.
Kinetic data
The thermodynamic activation parameters of decomposition processes of dehydrated complexes namely activation energy (E*), enthalpy (ΔH*), entropy (ΔS*) and Gibbs free energy change of the decomposition (ΔG*) were evaluated graphically by employing the Coats–Redfern relation [24]. The entropy of activation (ΔS*), enthalpy of activation (ΔH*) and the free energy change of activation (ΔG*) were calculated using the following equations
The data are summarized in Table 4. The activation energies of decomposition were found to be in the range 17.24–241.0 kJ mol−1. The high values of the activation energies reflect the thermal stability of the complexes [25–27]. The entropy of activation was found to have negative values in all the complexes which indicate that the decomposition reactions proceed with a lower rate than the normal ones.
Antimicrobial activity
Antibacterial and antifungal activities of the NOR ligand and its complexes are carried out against the Escherichia coli (Gram −ve), Bacillus subtilis (Gram +ve) and antifungal (tricoderma and penicillium activities). The results of the antimicrobial test are given in Table 5 and shown in Fig. 5. The antimicrobial activity is estimated based on the size of inhibition zone around dishes. The complexes are found to have high activity against Bacillus subtilis and penicillium, whereas the Hg(II) complex is more active than the Zn(II) and Cd(II) complexes against tricoderma.
Structural interpretation
The structures of the complexes of NOR with Cd(II), Hg(II) and Zn(II) ions have been confirmed from the elemental analyses, IR, molar conductance, UV–vis, mass and thermal analysis data. Thus, from the IR spectra, it is concluded that NOR behaves as a monobasic bidentate ligand coordinated to the metal ions via the deprotonated carboxylate O and carbonyl groups. From the molar conductance data, it is found that the complexes are non-electrolytes. On the basis of the above observations, tetrahedral geometries are suggested for the investigated complexes. As a general conclusion, the investigated complexes structures can be given as shown below (Fig. 6).
References
Zaki A, Schreiber EC, Weliky I, Knill JR, Hubsher JA. Clinical pharmacology of oral cephradine. J Clin Pharmacol. 1974;14:118–26.
Klastersky J, Daneau D, Weerts D. Cephradine. Chemotherapy. 1973;18:191–204.
Anacona JR. Synthesis and antibacterial activity of some metal complexes of beta-lactamic antibiotics. J Coord Chem. 2001;54:355–65.
Lozano MJ, Borrás J. Antibiotic as ligand. Coordinating behavior of the cephalexin towards Zn(II) and Cd(II) ions. J Inorg Biochem. 1987;31:187–95.
Zhao A, Carraher CE, Barone G, Pellerito C, Scopelliti M, Pellerito L. Mössbauer investigation on organotin polyester amines containing ciprofloxacin. Polym Mater: Sci Eng. 2005;93:414–6.
Iqbal MS, Ahmad AR, Sabir M, Asad SM. Preparation, characterization and biological evaluation of copper(II) and zinc(II) complexes with cephalexin. J Pharm Pharmacol. 1999;51:371–5.
Sorenson JRJ. Copper chelates as possible active forms of the antiarthritic agents. J Med Chem. 1976;19:135–48.
Brown DH, Smith WE, Teape JW, Lewis AJ. Antiinflammatory effects of some copper complexes. J Med Chem. 1980;23:729–34.
Williams DR. The metals of life. London: Van Nostrand Reinhold; 1971.
Ruiz M, Perelló L, Ortiz R, Castiñeiras A, Maichle-Mössmer C, Cantón E. Synthesis, characterization, and crystal structure of [Cu(cinoxacinate)2]·2H2O complex: a square-planar CuO4 chromophore. Antibacterial studies. J Inorg Biochem. 1995;59:801–10.
Castillo-Blum SE, Barba-Behrens N. Coordination chemistry of some biologically active ligands. Coord Chem Rev. 2000;196:3–30.
Turel I, Leban I, Bukovec N. Crystal structure and characterization of the bismuth(III) compound with quinolone family member (ciprofloxacin). Antibacterial study. J Inorg Biochem. 1997;66:241–5.
Turel I, Golič L, Bukovec P, Gubina M. Antibacterial tests of bismuth(III)–quinolone (ciprofloxacin, cf) compounds against Helicobacter pylori and some other bacteria. Crystal structure of (cfH2)2[Bi2Cl10]·4H2O. J Inorg Biochem. 1998;71:53–60.
Yang P, Li JB, Tian YN, Yu KB. Synthesis and crystal structure of rare earth complex with Ciprofloxacin. Chin Chem Lett. 1999;10:879–80.
Wu G, Wang G, Fu X, Zhu L. Synthesis, crystal structure, stacking effect and antibacterial studies of a novel quaternary copper(II) complex with quinolone. Molecules. 2003;8(2):287–96.
Turel I, Leban I, Klintschar G, Bukovec N, Zalar S. Synthesis, crystal structure, and characterization of two metal-quinolone compounds. J Inorg Biochem. 1997;66:77–82.
Turel I, Gruber K, Leban I, Bukovec N. Synthesis, crystal structure, and characterization of three novel compounds of the quinolone family member (norfloxacin). J Inorg Biochem. 1996;61:197–212.
Turel I, Leban I, Zupancic M, Bukovec N, Gruber K. An adduct of magnesium sulfate with a member of the quinolone family (ciprofloxacin). Acta Crystallogr C. 1996;52:2443–5.
Chen Z-F, Xiong R-G, Zuo J-L, Guo Z, You X-Z, Fun H-K. X-Ray crystal structures of Mg2+ and Ca2+ dimers of the antibacterial drug norfloxacin. J Chem Soc Dalton Trans. 2000;4013–4014.
Al-Mustafa J. Magnesium, calcium and barium perchlorate complexes of ciprofloxacin and norfloxacin. Acta Chim Slov. 2002;49:457–66.
Ruíz M, Perelló L, Server-Carrió J, Ortiz R, García-Granda S, Díaz MR, et al. Cinoxacin complexes with divalent metal ions. Spectroscopic characterization. Crystal structure of a new dinuclear Cd(II) complex having two chelate-bridging carboxylate groups. Antibacterial studies. J Inorg Biochem. 1998;69:231–9.
Gupta R, Saxena RK, Chaturvedi P, Virdi JS. Chitinase production by Streptomyces viridificans: its potential in fungal cell wall lysis. J Appl Bacteriol. 1995;78:378–83.
Geary WJ. The use of conductivity measurements in organic solvents for the characterisation of coordination compounds. Coord Chem Rev. 1971;7:81–122.
Coats W, Redfern JP. Kinetic parameters from thermogravimetric data. Nature. 1964;201:68–9.
Omar MM. Spectral, thermal and biological activity studies on ruthenium(II) complexes with some pyridylamines. J Therm Anal Calorim. 2009;96:607–15.
Rotaru A, Goşa M, Rotaru P. Computational thermal and kinetic analysis. Software for non-isothermal kinetics by standard procedure. J Therm Anal Calorim. 2008;94:367–71.
Verma RK, Verma L, Bhushan A, Verma BP. Thermal decomposition of complexes of cadmium(II) and mercury(II) with triphenylphosphanes. J Therm Anal Calorim. 2007;90:725–9.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Refat, M.S., Mohamed, G.G., de Farias, R.F. et al. Spectroscopic, thermal and kinetic studies of coordination compounds of Zn(II), Cd(II) and Hg(II) with norfloxacin. J Therm Anal Calorim 102, 225–232 (2010). https://doi.org/10.1007/s10973-009-0404-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-009-0404-x