Abstract
Non-isothermal decomposition of iron (III)-diclofenac anhydrous salt was investigated by thermogravimetry (TG) under different conditions in opened and closed α-alumina pans under nitrogen atmosphere. To estimate the activation energy of decomposition, the Capela and Ribeiro isoconversional method was applied. The results show that due to the lid cover different activation energies were obtained. From these curves a tendency can be seen where the plots maintain the same profile for closed lids and almost run parallel to each other. Independently of the different experimental conditions no remarkably different results have been obtained.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Several studies have been carried out on the metal diclofenac compounds [1–5], which focus on their anti-inflammatory activity, thermal behavior and spectroscopic characterization. Fini et al. [3] studied by thermogravimetry (TG) and differential scanning calorimetry (DSC) the diclofenac salts of one alkaline and two alkaline earth cations where the nature of the salts was examined. Different hydrate forms and, especially for divalent magnesium compound crystalline transition was reported. Bucci et al. [4] reported studies on divalent metal ions with diclofenac, which mainly consist of the determination of stoichiometry from TG curves and other spectroscopic data showing that the drug forms complexes with the metal ions. However, the experiments carried out by these authors, only mention the results of the thermal behavior but without showing all thermoanalitical curves. The iron(III)-diclofenac compound (Fe(Diclof)3) was also investigated by using spectroscopic techniques as well as the biological activity of the administered drugs [5].
The aim of this work is to investigate the thermal decomposition of Fe(Diclof)3 under non-isothermal circumstances, which was focused mainly on kinetic behavior. The experiments were carried out at different heating rates and two sample sizes, as well as in opened and crimped aluminum pan, in order to determine the effect of changed experimental conditions on the kinetic behavior and thus to obtain reference values of activation energy. Furthermore, the isoconversional method is used as a tool to obtain reliable and consistent kinetic information without the use of explicit kinetics models [6]. Thus, the activation energy (Ea/kJ mol−1) data were obtained applying the isoconversional method proposed by Capela et al. [7].
Kinetic theory
In kinetic analysis under non-isothermal conditions it can be considered the integral kinetic equation, defined by
where \( \beta = {{dT} \mathord{\left/ {\vphantom {{dT} {dt}}} \right. \kern-\nulldelimiterspace} {dt}} \) is a constant heating rate (T is the temperature and t is the time), g(α) is the integral form of the reaction model as a function of the extent of reaction α, A is the pre-exponential factor, E is the activation energy and R is the gas constant.
Kinetic parameters are estimated by fitting Eq. 1 to experimental data. As a consequence, the evaluation of the integral on the right side of Eq. 1 known as temperature integral is required. A difficulty arises from the fact that this integral does not have an exact analytical solution. Thus, it is convenient to approximate the integral of temperature for some functions that yield suitable estimates to these kinetic parameters.
In this work, the kinetic parameters are obtained using an approximation to the temperature integral based on the convergent of a Jacobi fraction proposed by Capela et al. [7]. This approximation is a rational function, given by the following equation:
A characteristic experimental curve presents the conversional fraction, α, as a function of the temperature for a given heating rate, β. For each fixed value of α there are corresponding values T α for temperature, values T α for activation energy and values A α for pre-exponential factor.
Replacing the integral in Eq. 1 by the approximation given in Eq. 2 the following expression is obtained for heating rate β as function of the \( x_{\alpha } = {{10^{3} } \mathord{\left/ {\vphantom {{10^{3} } {RT_{\alpha } }}} \right. \kern-\nulldelimiterspace} {RT_{\alpha } }} \):
where the activation energy is in kJ/mol and the parameter B α is defined as:
The estimates of the E α and B α can be obtained by the non-linear fitting of Eq. 3 to the β values as a function of x α.
Once the g(α) function has been determined for each conversional fraction, α, the estimation of the Arrhenius pre-exponential factor can be obtained from Eq. 4 and is given by following equation:
Experimental
The Fe(Diclof)3 compound was prepared by the stoichiometric addition of iron (III) chloride to an aqueous solution of potassium diclofenac salt. The obtained precipitate was filtered, washed with distilled water, dried at 25 °C and stored in a desiccator over anhydrous calcium chloride to constant mass.
Simultaneous TG-DTA curves were obtained on SDT 2960 model, from TA Instruments. The stoichiometry was obtained using around 7 mg sample mass in an α-alumina crucible and heating rates of 20 °C min−1 in synthetic air with flow of 100 mL min−1. The kinetic study parameters were obtained using several heating rates (5, 10 and 20 °C min−1) from 30 to 400 °C. Powdered samples of 2 and 5 mg (±0.05 mg) were placed to an α-alumina pan with and without cover and under a nitrogen flow of 100 mL min−1.
The diffraction patterns were obtained on a Siemens model D-500 X-ray diffractometer, using CuKα radiation (λ = 1.54 Å) and settings of 40 kV and 30 mA. This is compound was also examined using Scanning Electron Microscopy (SEM). The particles were sputter coated with a thin and uniform layer of gold using a vacuum evaporator and then examined using a Jeol model JSM-T-330A microscope at an accelerating voltage of 20 kV.
Results and discussion
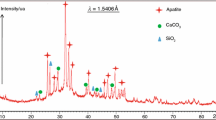
The X-ray powder diffraction patterns show that the compound is non-crystalline as it can be seen in Fig. 1a and b shows the SEM image of this compound, which is characterized by irregular shapes with different sizes, showing no regular or characteristic crystalline structures.
Simultaneous TG/DTA and DTG curves of this compound are shown in Fig. 2a. The first mass loss (1.1%) between 50 and 115 °C can be attributed to the loss of adsorbed water. The dry compound is stable up to 185 °C and above this temperature the thermal decomposition occurs in two main steps. The absence of a large exothermic peak for the first decomposition is probably due the existence of equilibrium between the exothermic and endothermic process, which present only one small exothermic peak as a result of these simultaneous reactions. The second exothermic peak can be attributed to the oxidation of the remainder of the ligand. Calculation confirmed the formation of Fe2O3.
Figure 2b shows the TG curves for Fe(Diclof)3 in nitrogen atmosphere at a heating rate of 5, 10 and 20 °C min−1 with 5 mg of initial sample mass in an opened pan. As observed, the thermal decomposition takes place progressively in single steps between 190 and 350 °C. TG curves for other conditions (open and closed pan) also possess the same profile. The kinetic parameter of this compound was obtained for the first decomposition step.
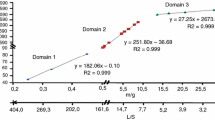
Figure 3a shows the distribution of the heating rate, β, as a function of the 1000/RT α for conversion degree of 0.90 for 5 mg sample mass in an open pan. It can be observed the adjustment variation for E1 values, which correspond to the fitting for the activation energy, proposed by Capela et al. [7].
The average values of the activation energy (E a) of the kinetics data for all compounds are shown in Table 1, where the resulting correlation coefficient has a good close linear fit (r).
The activation energy (E a) versus conversion degree (α) values, are shown in Fig. 3b. Thus, this difference was associated to the heat conductivity of the cover on the sample, etc., which lead to changes in the reactions [8–12]. From these curves a tendency can be seen where the plots maintain the same contour for covered lids and almost run parallel to each other. It indicates that both the analysis conditions have the same behavior, independent of the sample utilized. Furthermore, by using the same sample masses applying open or closed lids differences between the E a versus α plots have been observed. The closed pan sample promotes a decrease in activation energy, which is probably because of the heat of the reaction, which will favor the decomposition step. However, for open pan, activation energy has the same tendency but its numerical value is higher. This fact is attributed to the flowing nitrogen over the sample during the decomposition step, which easily dissipates the heat generated and consequently cools down the sample.
Conclusions
The presented results clearly showed that the opened or covered sample pan as well as the different sample masses has influence on the activation energy (E a) values. In addition, in a previous work [11] for basic zinc carbonate it was observed that the activation energy continuously varies as function of the degree of conversion as well as decreases with the increase of sample size, which was attributed to the increase in the reaction temperature. In our case when the studied compound is more complex compared to zinc carbonate. Therefore, these data suggest that the activation energy increases with the increase of sample mass in an open crucible.
References
Konstandinidou M, Kourounakis A, Yiangou M, Hadjipetrou L, Kovala-Demertzi D, Hadjikakou S, et al. Anti-inflammatory properties of diclofenac transition metalloelement complexes. J Inorg Biochem. 1998;70:63–9.
Kovala-Demertzi D. Transition metal complexes of diclofenac with potentially interesting anti-inflammatory activity. J Inorg Biochem. 2000;79:153–7.
Fini A, Fazio G, Rosetti F, Holgado MA, Iruin A, Alvarez-Fuentes J. Diclofenac salts. III. Alkaline and earth alkaline salts. J Pharm Sci. 2005;94:2416–31.
Bucci R, Magrı AD, Magrı AL, Napoli A. Spectroscopic characteristics and thermal properties of divalent metal complexes of diclofenac. Polyhedron. 2000;19:2515–20.
Kugtrin SA, Zivanovic L, Zecevic M, Radulovic D. Spectrophotometric study of diclofenac-Fe(III) complex. J Pharm Biomed Anal. 1997;16:147–53.
Vyazovkin S, Wight CA. Model-free and model-fitting approaches to kinetic analysis of isothermal and nonisothermal data. Thermochim Acta. 1999;340/341:53–68.
Capela JMV, Capela MV, Ribeiro CA. Rational approximations of the Arrhenius integral using Jacobi fractions and gaussian quadrature. J Math Chem. 2009;45:769–75.
Galwey AK, Brown ME. Thermal decompositions of ionic solids. 1st ed. Elsevier: Amsterdam; 1999.
Guinesi LS, Ribeiro CA, Crespi MS, Santos AF, Capela MV. Titanium(IV)–EDTA complex. J Therm Anal Calorim. 2006;85:301–7.
Lima EN, Crespi MS, Ribeiro CA, Almeida S. Non-isothermal kinetic for lyophilized leachate from sanitary landfill and composting usine. J Therm Anal Calorim. 2007;90:823–6.
Liu Y, Yu J, Zhao J, Zhang H, Deng Y, Wang Z. Simultaneous thermal analysis of a cobalt(II) complex with nicotinate. Thermochim Acta. 2004;419:115–17.
Prasad TP, Kanungo SB, Ray HS. Non-isothermal kinetics: some merits and limitations. Thermochim Acta. 2002;203:503–14.
Acknowledgements
CAPES/CNPQ and IPT – Instituto de Pesquisas Tecnológicas do Estado de São Paulo by TG curves.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kobelnik, M., Bernabé, G.A., Ribeiro, C.A. et al. Decomposition kinetics of iron (III)-diclofenac compound. J Therm Anal Calorim 97, 493–496 (2009). https://doi.org/10.1007/s10973-009-0336-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-009-0336-5