Abstract
The pyrolysis and combustion of cellulosic substances treated with MAP and DAP have been studied using thermal analysis, flame spread tests and a specifically designed apparatus for smoldering combustion test. The samples used were: cotton string, cotton fabric and pure cellulose powder. Diammonium Phosphate (DAP) and Monoammonium Phosphate (MAP) can reduce the combustion and pyrolysis maximum mass loss temperature, decrease the initial pyrolysis temperature and considerably increase mass residue. Moreover, MAP and DAP reduce the flaming combustion rate of cellulosic materials and completely inhibit smoldering combustion. This study can facilitate a better understanding of the mechanism of pyrolysis and combustion of fire-retarded cellulosic materials.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cellulosic materials are unstable at temperatures above 200 °C, producing a range of small, volatile, organic compounds which are highly inflammable. These thermal degradation products evolve large amounts of heat, which causes further degradation and burn. A simple model of the burning process of cellulosic materials is summarised in Fig. 1 [1, 2]. For continuous burning, sufficient heat must be applied to decompose the material and ignite the products of degradation, and sufficient heat from the combustion must be transferred back to the cellulosic matter to maintain the cycle when the heat is withdrawn. Obviously, a fire retardant should break the cycle at one or more of the points 1, 2, 3, 4 or 5 (Fig. 1).
Fire retardants are defined as chemicals that modify pyrolysis reaction of polymers or oxidation reactions implied in the combustion by slowing down or hindering the burning process. The fire retardant can act in various ways, i.e. physically or chemically, or both. In fact, many types of fire retardants are used in consumer products such as inorganic fire retardants, which are mainly phosphorus, antimony, aluminum and boron-containing compounds, chlorides and bromides, etc. [3].
A number of scientists have investigated the fire behavior of different cellulosic products using thermal analysis techniques [4–6] and have suggested various kinetic models for their decomposition [7, 8]. The fire behavior of cellulosic materials, treated with different kind of additives has been also examined by analytical techniques [9–12]. However the small samples used in most of the above analytical methods and the rapid removal of pyrolysis and combustion products can lead to an erroneous interpretation in terms of ignitability performance in field conditions. Therefore, the information provided by analytical methods should be supported by lab-scale tests, approaching the actual fire conditions.
The objective of the present work is to evaluate the fire retarding properties of phosphorus compounds (DAP and MAP) on the following flammability properties of cellulosic materials (i.e. cotton fabric, cotton string, and pure cellulose): (1) pyrolysis, (2) smoldering combustion, (3) flaming combustion. For this purpose, a combination of analytical and bench-scale techniques was selected: (1) Classical thermogravimetry (TG, DTG, DTA) for the pyrolysis study, (2) a lab-scale DTA apparatus, specifically designed for testing the smoldering combustion study, (3) flame spread tests for the flaming combustion study. This research can facilitate the fire management for confronting domestic, forest and industry (i.e. fabric, wood, paper) fires.
Experimental
Samples
The samples used were: 100% cotton fabric with area density 175 g m−2, 100% cotton strings and α-cellulose powder of purity 99.5%. Cellulose was a Fluka p.a. reagent with a mesh size <250 μm. The chemical retardants (NH4)2HPO4 (DAP) and NH4H2PO4 (MAP) were Merck laboratory reagents with purity ≥98.0% and particle size less than 250 μm.
The cotton fabric and cotton string samples were first dried at 50 °C for 24 h and then were baptized into aqueous DAP and MAP solutions in order to achieve 5% and 10% mass/mass retardant concentration on cellulosic materials after drying the samples.
The cellulose retardant samples were prepared by adding and mixing well a certain quantity of retardant (DAP, MAP) in fine powdered form with a certain quantity of cellulose (5% and 10% mass/mass).
The uniformity of all samples prepared was checked by optical microscope. Then, the samples were stored for at least 48 h in a conditioning box, at 31.3 °C and relative humidity 8%. Thus, the equilibrium moisture content of the samples retained 2%, according to the insertion tables given in the literature [13].
Thermal analysis
Each sample, weighing around 15–17 mg, was introduced in an open type alumina sample holder. The experiments were carried out under nitrogen atmosphere condition (with a flow rate of 100 mL min−1) and a linear heating rate of 10 °C min−1 from 25 to 600 °C, using a Mettler TGA/SDTA 851 module, supported by a PC and software for control and data handling. The heating rate was relatively low in order to achieve high resolution on DTG/SDTA curves and to ensure a minor deviation between sample and oven temperature.
Lab-scale smoldering combustion test
The lab-scale flammability tests were performed using a specifically designed apparatus for monitoring the forest species temperature, under precisely controlled temperature and static air atmosphere conditions. A detailed description of the apparatus and the operating conditions used are given in previous reports [14, 15]. The 8 cm3 cubic sample holder was filled with 2.5–3.0 g sample with particle size 0.1–0.2 mm. The oven temperature was increased from 20 to 500 °C with a low heating rate of 0.5 °C min−1, to favor smoldering combustion. Oven and sample temperatures were recorded every 5 s, whereas the initial combustion temperature and combustion duration were determined, based on the first derivative curves of sample temperature profiles [14].
Combustion spread rate test
A detailed description of the apparatus and the experimental conditions used is given in previous work [16–18].
The samples were placed over a thermal lark and by applying a pilot flame, we measured both the flaming combustion time and the smoldering combustion time. The measurements have been repeated by holding cotton strings or fabric of 20 cm length vertically from a steady point inside a ventilation duct in order to evaluate the influence of sample direction on burning.
A large number of experiments (i.e. 30–60) was conducted for each sample: untreated cotton string, untreated cotton fabric, cotton string treated with DAP (5%, 10% mass/mass) and MAP (5%, 10% mass/mass), cotton fabric treated with DAP (5%, 10% mass/mass) and MAP (5%, 10% mass/mass). Experiments with cellulose powder could not be performed, due to sample constraints, caused by its granular form.
Results and discussion
Thermal analysis
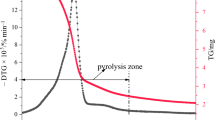
In Fig. 2 the TG/DTG/SDTA graphs for pure and retardant treated cotton fabric are shown. Similar graphs were obtained for the cotton fabric and cellulose samples. The data derived from the TG/DTG analyses of all samples are presented in Table 1.
According to previous works [2–4] the pyrolysis of cellulosic materials includes three stages: initial, main, and char decomposition.
-
1.
In the initial stage, at temperature below 312 °C for string and cellulose and below 340 °C for fabric (Table 1), the most important changes are physical property changes followed by mass loss. At this stage the amorphus region of cellulosic matter is deformed.
-
2.
The main pyrolysis stage occurs at 312–379 °C for string, 314–348 °C for cellulose and 342–375 °C for fabric samples (Table 1). In this stage, both the mass loss and mass loss rate are high and most of pyrolysis products are produced (l-glucose and combustible gases). At this stage the crystalline region of cellulose is decomposed.
-
3.
The char pyrolysis occurs at the temperature above 380 °C (Table 1), where the mass loss rate is low. During this process, dewatering, decarboxylization and charring reactions take place, producing double bond, l-glucose, carboxyl and carbonyl products. Thus, the carbon content in the decomposed products becomes higher and higher, and charred residues are formed.
Similarly, the pyrolysis of cellulosic materials treated with fire retardants occurs in three stages but at lower temperatures and with lower mass losses than those obtained with the untreated samples. This observation is especially recorded for the 10% MAP treated samples (Fig. 2).
In particular, the recorded mass residues at 600 °C for the untreated samples of cotton string, cotton fabric and cellulose were 22.5%, 17.7% and 14% respectively (Table 1, column 3), whereas for the 10% MAP treated samples were 39%, 36% and 29% respectively (Table 1, column 3). The samples treated with 5% MAP, 5% DAP and 10% DAP have an intermediate mass loss (Table 1, column 3). Thus, the retardants examined promote the formation of solid residues (Fig. 1, action 4).
For the determination of the net mass residue of the treated substances, the residue of pure retardant has been subtracted from the total residue at the same temperature.
Furthermore, the presence of retardants decreases the initial pyrolysis temperature. The maximum decrease is caused by MAP. Thus, by subtracting the retardant-treated initial pyrolysis values from the untreated ones and dividing by the untreated initial pyrolysis values, it can be calculated that 10% MAP addition causes a 28% decrease for cotton fabric, 18% for cotton string and 10% for pure cellulose (Table 1, column 1). In Table 1 we can also see a considerable shift of the temperature of the maximum mass loss rate to lower values.
The above shifts to lower temperatures cause an earlier liberation and dissipation of flammable gases, before their ignition temperature is reached [19]. This is another parameter beyond those reported in Fig. 1, which explains the function of the fire retardants examined.
Finally, the DTA scan of cotton fabric reveals a main endothermic peak at 340–370 °C, which is displaced at lower temperatures as the retardant concentration increases (Fig. 2). Similar observations were recorded for cellulose and string samples.
Apparatus for testing smoldering combustion properties of materials
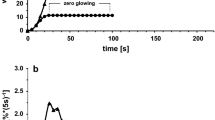
Under the experimental conditions employed in this study (very low heating rate and static air atmosphere) a smoldering type of combustion took place. Figure 3 shows the sample temperature-time profiles of treated and untreated samples. The relative data derived from this analysis (i.e., initial combustion temperature, mass residue) are presented in Table 2.
The fire-retardants tested reduce the relative initial smoldering combustion temperature of cellulosic materials, especially when 10% mass/mass was applied (Table 2, column 1).
In addition, the recorded mass residues at 500 °C for the untreated samples of cotton string, cotton fabric and cellulose were 2%, 3% and 1% respectively (Table 2, column 3), whereas those for the 10% MAP treated samples were 7%, 13% and 12% respectively (Table 2, column 3). The samples treated with 5% MAP, 5% DAP and 10% DAP have intermediate mass residue values (Table 2, column 3). The net mass residues were determined with the same method applied in 3.2. However, the remarkable difference between the mass residue values reported in Table 1 and in Table 2 is attributed to the fact that values in Table 1 refer to pyrolysis, whereas those in Table 2 to combustion. Concluding, the retardants examined promote char formation in smoldering combustion (Fig. 1, action 4).
Combustion spread rate test (flaming and smoldering)
A large number of experiments (i.e. 30 measurements) was conducted for retardant treated and untreated cotton fabric and cotton string samples to determine the combustion spread rate. The results were statistically processed, using the Q-test method at a 95% confidence level and the average values are shown in Table 3.
Table 3 shows that 5% DAP reduces the upward vertical flaming combustion rate of cotton string almost by 45% and this effect increases as the retardant concentration increases to 10%. Similar results were obtained with MAP treated samples. In contrast, relatively low effect of MAP 5% on the vertical flame spread rate of cotton fabric was recorded (Table 3). Moreover, treatment with both DAP and MAP inhibits smoldering combustion completely, which affirms the results from the smoldering combustion tests.
In agreement with the experimental data presented in sections "Thermal analysis" and "Apparatus for testing smoldering combustion properties of materials", mass residue increases up to 12% when cotton materials are treated with 5% DAP and up to 15% with 5% MAP (Table 3). Similarly at higher concentration (10% mass/mass) the mass residue was increased up to 15%.
Regarding horizontal flame spread process, cotton fabric exhibits both flaming and smoldering combustion at very low rates, whereas cotton string burns only with smoldering combustion. Treatment with DAP and MAP prohibits the combustion of tested materials, under the examined conditions.
Retardancy mechanism
The retardancy effect of DAP and MAP can be explained by their thermal decomposition, which takes place according to the following scheme [20, 21]:
During decomposition a significant amount of heat is absorbed (Fig. 1, action 1). Both diammonium and monoammonium phosphates are easily converted to H3PO4 because the ammonia cations (NH4 +) are driven off at low temperatures. In the presence of phosphoric acid, some of the hydroxyl groups of the cellulose are esterified. Ester decomposition occurs at higher temperatures to form a double bond and an acid molecule, which becomes available for further reaction. By successive esterification and elimination reactions, stable conjugated structures are built up. Conjugated structures of this kind are the precursors for the formation of char. Consequently, smaller amounts of flammable products are formed (Fig. 1, action 4). In addition, the relatively large amount of water formed will tend to quench the flame (Fig. 1, action 3). Also it is reported that cellulose will be protected from the heat of combustion by the layer of char formed on its surface (Fig. 1, action 1) [2, 10].
Conclusions
The main conclusions of this study can be summarized as follows:
-
1.
Using a combination of simple, low-cost and not time-consuming tests, we were able to evaluate accurately most aspects of the burning behaviour of various common cotton products.
-
2.
The addition of MAP and DAP to cellulosic materials lowers their initial pyrolysis temperature and the temperature of the maximum mass loss rate. Thus, an earlier liberation of flammable gases takes place, before their ignition temperature is reached, enhancing the pyrolysis temperature. Their effect increases as the concentration level of the retardant increases and MAP is overall more efficient than DAP.
-
3.
DAP and MAP also increase the pyrolysis and smoldering combustion mass residue of all kinds of cellulosic materials. Thus, they increase char formation (Fig. 1, action 4).
-
4.
Based on the combustion spread rate experiments and the smoldering combustion tests, we found that both DAP and MAP are very effective glow inhibitors and reduce the flame spread rate of cellulosic materials by half, even in upward burning tests. They eliminate completely smoldering combustion in vertical and horizontal flame spread tests and they reduce the ignition delay time.
-
5.
The retardant efficiency increases as the retardant concentration increases, although not proportionally. In some experiments MAP was found more efficient whereas in some other, DAP was found to be more efficient. This leads to the conclusion that the highest overall efficiency could be obtained by chemical mixtures.
-
6.
The above conclusions are valuable to fire management research, especially in forest fire research, and can facilitate the development of new fire-fighting agents for confronting domestic, forest and industrial fires.
References
Horrocks AR. An introduction to the burning behavior of cellulosic fibres. J Soc Dye Color. 1983;99:191–7.
Price D, Horrocks AR, Akalin M, Faroq AA. Influence of flame retardants on the mechanism of pyrolysis of cotton (cellulose) fabrics in the air. J Anal Appl Pyrolysis. 1997;40/41:511–24.
Mostashari SM, Mostashari SZ. Combustion pathway of cotton fabrics treated by ammonium sulfate as a flame-retardant studied by TG. J Therm Anal Calorim. 2008;91(2):437–41.
Zhu P, Sui S, Wang B, Sun K, Sun G. A study of pyrolysis and pyrolysis products of flame-retardant cotton fabrics by DSC, TGA, and PY–GC–MS. J Anal Appl Pyrolysis. 2004;71(2):645–55.
Drevelle C, Lefebvre J, Duquesne S, Le Bras M, Poutch F, Vouters M, et al. Thermal and fire behaviour of ammonium polyphosphate/acrylic coated cotton/PESFR fabric. Polym Degrad Stab. 2005;88(1):130–7.
Tian CM, Guo HZ, Zhang HY, Xu JZ, Shi JR. Study on the thermal degradation of cotton cellulose ammonium phosphate and its metal complexes. Thermochim Acta. 1995;253:243–51.
Moltó J, Font R, Conesa JA, Martín-Gullón I. Thermogravimetric analysis during the decomposition of cotton fabrics in an inert and air environment. J Anal Appl Pyrolysis. 2006;76(1–2):124–31.
Lecoeur E, Vroman I, Bourbigot S, Lam TM, Delobel R. Flame retardant formulations for cotton. Polym Degrad Stab. 2001;74(3):487–92.
Davies D, Horrocks AR, Greenhalgh M. Ignition studies on cotton cellulose by DTA. Thermochim Acta. 1983;63(3):351–62.
Wanna JT, Powell JE. Thermal decomposition of cotton cellulose treated with selected salts. Thermochim Acta. 1993;226:257–63.
Bourbigot S, Chlebicki S, Mamleev V. Thermal degradation of cotton under linear heating. Polym Degrad Stab. 2002;78(1):57–62.
Horrocks AR, Kandola BK, Davies PJ, Zhang S, Padbury SA. Developments in flame retardant textiles—a review. Polym Degrad Stab. 2005;88(1):3–12.
ASTM D 4933-91 (reapproved 1997) Standard guide for moisture conditioning of wood and wood-based materials.
Liodakis S, Vorisis D, Agiovlasitis IP. A method for measuring the relative particle fire hazard properties of forest species. Thermochim Acta. 2005;437(1–2):150–7.
Liodakis S, Vorisis D, Agiovlasitis IP. Testing the retardancy effect of various inorganic chemicals on smoldering combustion of Pinus halepensis needles. Thermochim Acta. 2006;444(2):157–65.
Official Journal of the European Communities 79/831/EEC (reapproved 2003), No. L251, 19.9.84, p. 86.
Liodakis S, Kakardakis T. Measuring the relative particle foliar combustibility of WUI forest species located near Athens. J Therm Anal Calorim. 2008;93(2):627–35.
Liodakis S, Antonopoulos I, Agiovlasitis IP, Kakardakis T. Testing the fire retardancy of Greek minerals hydromagnesite and huntite on WUI forest species Phillyrea latifolia L. Thermochim Acta. 2008;469(1–2):43–51.
Liodakis S, Bakirtzis D, Dimitrakopoulos AP. Autoignition and thermogravimetric analysis of forest species treated with fire retardants. Thermochim Acta. 2003;399(1–2):31–42.
Gadalla AM, Abadir MF, Kasem MY, Salem FT. Kinetics of pyrolysis of some fire retardants and treated fabrics. AIChE J. 1984;30(1):50–5.
Liodakis SE, Statheropoulos MK, Tzamtzis NE, Pappa AA, Parissakis GK. The effect of salt and oxide-hydroxide additives on the pyrolysis of cellulose and Pinus halepensis pine needles. Thermochim Acta. 1996;278(1–2):99–108.
Acknowledgements
The authors acknowledge the Committee of Basic Research and particularly the Program PROTAGORAS for financially supporting the work. The project is co-funded by the European Social Fund (75%) and National Resources (25%).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liodakis, S., Fetsis, I.K. & Agiovlasitis, I.P. The fire-retarding effect of inorganic phosphorus compounds on the combustion of cellulosic materials. J Therm Anal Calorim 98, 285–291 (2009). https://doi.org/10.1007/s10973-009-0307-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-009-0307-x