Abstract
Four complexes of the nuclear structure NiII–ZnII were prepared with bis-N,N′-(salicylidene)-1,3-propanediamine (LH2), bis-N,N′-(salicylidene)-2,2′-dimethyl-1,3-propanediamine (LDMH2) and the reduced derivatives of these Schiff bases, bis-N,N′-(2-hydroxybenzyl)-1,3-propanediamine (LHH2), bis-N,N′-(2-hydroxybenzyl)-2,2′-dimethyl-1,3-propanediamine (LDMHH2). The complexes were characterized using IR spectroscopy, elemental analysis and thermogravimetric methods. The stoichiometry of the complex molecules were found to be NiL·ZnCl2·(DMF)2, NiLDM·ZnCl2·(DMF)2, NiLH·ZnCl2·(DMF)2 and NiLDMH·ZnCl2·(DMF)2. The molecular models of the complexes prepared with the reduced Schiff bases were determined according to the X-ray diffraction method. It is seen that in these complexes Ni(II) is in octahedral and Zn(II) is in tetrahedral coordination sphere. Ni(II) ion is coordinated between two nitrogen and two oxygen donors of the ligand and oxygen donors of the two DMF molecules. Zn(II) ion on the other hand is coordinated between two oxygen of the organic ligand forming two μ bonds. It also coordinates two Cl ions. The thermogravimetric analysis showed that the complex NiLDMH·ZnCl2·(DMF)2 containing methyl groups is more stable than the other complex NiLH·ZnCl2·(DMF)2 containing reduced Schiff base. The coordinative DMF molecules in NiLDMH·ZnCl2·(DMF)2 were thermally cleaved. However, the cleavage of DMF molecules NiLH·ZnCl2·(DMF)2 resulted in the thermal degradation of the complex. In order to explain the TG data of the ligands were titrated in non-aqueous medium and their basicity strengths were determined. It was found that the basicity of the ligands containing two methyl groups were stronger. It is understood that the two methyl groups increase the negative charge density on nitrogen causing an increase in complex stability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
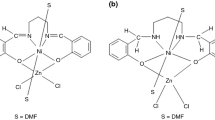
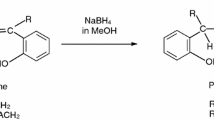
Bis-N,N′-(salicylidene)-1,3-propanediamine (LH2) is a ligand which is highly prone to forming multi nuclear complexes [1–4]. Ni(II) and Cu(II) complexes of this ligand have square planar coordination sphere and the molecular structures of these complexes determined using X-ray diffraction method are given in literature [5]. In the presence of a Lewis acid, these square planar complexes coordinate to the Lewis acid from their phenolic oxygens and form binuclear complexes [6–11]. Ni(II) and Cu(II) ions in these binuclear complexes can also be coordinated with a nitrogen containing ligand such as Py or a solvent such as DMF [12–14]. Bis-N,N′-(salicylidene)-1,3-propanediamine (LH2) is easily reduced with NaBH4 in amphiprotic medium and turn into an ONNO type phenol–amine ligand [15]. In this study, bis-N,N′-(salicylidene)-1,3-propanediamine (LH2) and bis-N,N′-(salicylidene)-2,2′-dimethyl-1,3-propanediamine (LDMH2) are reduced to obtain bis-N,N′-(2-hydroxybenzyl)-1,3-propanediamine (LHH2), bis-N,N′-(2-hydroxybenzyl)-2,2′-dimethyl-1,3-propanediamine (LDMHH2). Heterodinuclear complexes are obtained from both the Schiff bases (LH2 and LDMH2) and the reduced Schiff bases (LHH2 and LDMHH2) in the presence of ZnCl2 in DMF medium. The formulas of the ligands LH2, LDMH2, LHH2 and LDMHH2 are given in Fig. 1a and b.
The molecular models of the complexes similar to I (NiL·ZnCl2·(DMF)2) and II (NiLDM·ZnCl2·(DMF)2) are reported in literature [16, 17]. The X-ray diffraction studies of the complexes III (NiLH·ZnCl2·(DMF)2) and IV (NiLDMH·ZnCl2·(DMF)2) which gave suitable crystals were carried out and the complexes I–IV were thermogravimetrically analyzed. The four complexes prepared were characterized with IR and elemental analyses. They were later studied thermogravimetrically. The two Schiff base ligands; bis-N,N′-(salicylidene)-1,3-propanediamine, bis-N,N′-(salicylidene)-2,2′-dimethyl-1,3-propanediamine and the two reduced Schiff bases; bis-N,N′-(2-hydroxybenzyl)-1,3-propanediamine,bis-N,N′-(2-hydroxybenzyl)-2,2′-dimethyl-1,3-propanediamine were titrated with HClO4 in acetonitrile solvent to determine their relative basicity strengths in order to explain the TG data.
Experimental
Chemicals
1,3-diaminopropane (Merck), 2,2′-dimethyl-1,3-propanediamine (Merck), salisylaldehyde (Fluka), ethanol (Riedel-deHaën), methanol (Riedel-deHaën), nickel(II) chloride hexahydrate (Aldrich), zinc(II) chloride (Merck), sodium boronhydride (Fluka), acetonitrile (Riedel-deHaën), perchloric acid (Merck) were used without further purification.
Preparation of the ligands
Schiff bases LH2 and LDMH2 were prepared by the condensation reaction of 1,3-diaminopropane, 2,2′-dimethyl-1,3-propanediamine and salicylaldehyde in ethanol. They were recrystallised in 1:1 EtOH:H2O mixture. LH2; melting point 58–59 °C, reaction yield >90%, color yellow; LDMH2; melting point 97–98 °C, reaction yield >95%, color yellow.
Approximately 3.0 g of these Schiff bases were dissolved in 50 mL MeOH by mixing and NaBH4 was added to this solution in drop wise manner until a colorless solution was obtained. The resulting solution was poured into approximately 250 mL icy water and kept on the bench for 24 h. The white colored organic precipitate formed at the end of this period is the reduced Schiff base. It was recrystallised from 70% EtOH. LHH2; melting point 106–108 °C, reaction yield >75%, color white; LDMHH2; melting point 95–96 °C, reaction yield >90%, color white.
Preparation of the complexes
Four complexes were prepared using the same method. 0.001 mol ligand (LH2, LDMH2, LHH2, LDMHH2) was solved in 40 mL hot DMF. Solution of 0.2 mL Et3N and 0.001 mol (0.238 g) NiCl2·6H2O in 0.20 mL hot MeOH was added to this solution. Afterwards solution of 0.001 mol (0.136 g) ZnCl2 in 10 mL hot MeOH was added and the mixture thus prepared was kept on the bench for 2–3 days. The crystals which precipitated at the end of this period were filtered and dried in air.
Measurement techniques
C, H, N element analysis of the complexes were made on Leco 932 CHNS analyser. Ni and Zn analysis were made on GBC Avanta PM AAS equipment. Chlorine analysis was made gravimetrically with the help of AgNO3. IR spectra were obtained on a Perkin Elmer Spectrum 100 FTIR spectrometer using the ATR attachment.
Thermogravimetric analyses were carried out on Shimadzu DTG-60 H equipment in Pt pans under N2 atmosphere. Heating rate was 10 °C/min. Curves were recorded between 30 and 750 °C. The pans were cleaned with O2 at 750 °C.
All potentiometric measurements were carried out in an 80-mL jacketed titration cell thermostated at 25.0 ± 0.1 °C and under nitrogen atmosphere. An Orion 960 automatic titrator, equipped with a combined pH electrode (Ingold) containing a filling solution of 0.1 M NaCl was used for measuring the cell emf values. Titration of the ligands was carried out in MeCN and 0.034 M HClO4 was used as titrant.
The intensity data were collected at room temperature using an Enraf-Nonius CAD 4 diffractometer [18] with MoKα radiation with ω/2θ scan mode. The cell parameters were determined from a least-squares refinement of 25 centered reflections in the range of 2.46 ≤ θ ≤ 23.41° in III, 2.12 ≤ θ ≤ 26.29° in IV. Three standard reflections that were periodically measured for every 120 min during data collection showed no significant intensity variations. The ranges of h, k, l are 0 ≤ h ≤ 15, 0 ≤ k ≤ 21, 0 ≤ l ≤ 23 in III, 0 ≤ h ≤ 11, −12 ≤ k ≤ 12, −22 ≤ l ≤ 21 in IV, respectively. There were 4,422 reflections were collected for complex III 3572 of were utilized, the number of reflections collected for complex IV were 4,081 of which 2,898 were utilized for structural determinations and refinement according to I ≥ 2σ(I). Cell refinement and data reduction were carried out using CAD4 Express [18]. The structures were solved by direct method using the program SHELXS97 and refined using SHELXL97 [19] in the WinGX package [20]. All non-hydrogen atoms were refined isotropically and then anisotropically by full matrix least squares method. All the hydrogen atoms were placed geometrically and refined as riding with Ueq(H) = 1.2Uiso(C).
Results and discussion
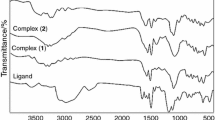
Important IR data and elemental analysis results of the ligands and complexes are given in Table 1. The data in Table 1 are in agreement with the formulas given in Fig. 1a and b. The band seen at 1,652 cm−1 in the IR spectra is the stretching vibration of the carbonyl group in DMF. νC=N stretching vibrations of complexes I and II are seen at 1,620 cm−1. In Schiff bases which contain an aliphatic carbon attached to the iminic nitrogen νC=N vibration band is observed at wave numbers bigger than 1,630 cm−1. However, it is a known fact that these vibration bands shift 10–25 cm−1 towards lower energy. These vibrations for ligands (LH2 and LDMH2) are observed at 1,642; 1,644 cm−1 have moved to lower energy by 20 cm−1. This shows that iminic nitrogens participate in coordination [21].
In complexes III and IV stretching bands are observed at νN–H 3,283–3,218 cm−1. These νN–H bands in ligands are observed at 3,326–3,268 cm−1. Since LH2 and LDMH2 complexes do not carry N–H bonds these bands are not observed in complexes I and II. However, when the ligand is reduced C=N group is convert to C–NH. In this case νN–H band becomes clearly visible and νC=N band disappears for complexes III and IV.
In the IR spectrum of the ligands only the phenolic stretching bands νO–H are observed at energies lower than expected. The reason for this is the strong hydrogen bond between the phenolic O–H and iminic and aminic nitrogen atoms. This hydrogen bond may cause the proton of the base to be transferred onto nitrogen atom and move the νO–H vibration to lower energy by 700–1,000 cm−1 [22]. In this study νO–H vibrations of the ligands are observed at 2,715–2,625 cm−1.
TG is one of the most efficient methods in determination of complex stoichiometries [23]. The TG curves of the complexes are given in Fig. 2a–d. Full thermoanalytical data of the complexes are given in Table 2.
Mass loss in the first thermal reactions of complexes I, II and IV is observed at temperatures between 135 and 240 °C. This mass loss corresponds to the two DMF molecules in the expected complex stoichiometry. In contrast, in complex III, termination of the first thermal reaction is observed clearly after decomposition is initiated at 203 °C. In complexes I and II ligands are Schiff bases whereas in complexes III and IV ligands are reduced Schiff bases. If the ligand is a Schiff base, DMF molecules start to break off at around 135 °C and the reaction is completed about at 200 °C. DTA peaks are observed around 161–177 °C. This is close to the results cited in literature [23]. If the ligand is reduced Schiff base (phenol amine) DMF break off takes place at higher temperatures. In complex IV, thermal reaction which starts at around 160 °C is completed at around 240 °C. In complexes I and II second thermal reaction starts around 380 °C and continues up to 650 °C. In complex IV the complex is stabile for by 35 °C after the completion of the DMF removal reaction at 230 °C. Second thermal reaction starts at around 270 °C and continues up to 700 °C. This second decomposition reaction of the complexes I, II and IV are similar, but their initiation temperatures are different. It is considered that in these reactions organic ligand start to decompose first and in parallel to the organic ligand’s decomposition ZnCl2 leaves the structure. O2 was added to the medium at around 750 °C and a strong exothermic peak appeared in all four curves. This exothermic peak is the result of the combustion of the carbon particles formed during decomposition of the ligand. The mass remaining after the formation of this peak corresponds to NiO as shown in Table 2. Curve of complex III is different from the other three curves. This complex remains unchanged up to 203 °C but starting from this temperature onward it undergoes a constant decomposition up to 700 °C. In this complex the upper limit where the DMF molecules leave the structure is unknown. From the curves in Fig. 2a–d we can suggest that complex III is formed with the DMF molecules alone. DMF molecules make coordination in other complexes as well but these complexes remain stable with Ni–Ligand–ZnCl2 nucleus after the DMF molecules are thermally separated. It is considered that in complex III Ni–ligand–ZnCl2 units also start to decompose with the separation of DMF. However, in complexes I, II and IV the complex remaining after the separation of the DMF molecules maintains its stability.
The complexes are formed by the coordination of ZnCl2 from the phenolic oxygens. Since ZnCl2 attracts the electrons of the phenolic oxygens, phenolic oxygens can transfer less electrons to Ni(II) and in turn Ni(II) ion coordinates DMF molecules to compensate this electron deficient. If the nitrogen atom of the organic ligand has sufficient amount of electron density, it is considered that the complex will maintain its existence even after the DMF molecules leave the structure. However, in the case of complex III the electron density on the aminic nitrogen atoms of the organic ligand is insufficient and the complex starts to decompose after the DMF molecules leave the complex structure. In order to understand charge density on the nitrogen atoms of LHH2 and LDMHH2 these ligands were potentiometrically titrated in MeCN. Potentiometric titration in non-aqueous solvents is one of the simplest methods that give an idea about the acidity and basicity strengths of organic acids and bases [24]. The basicity of organic matter can be determined from the half-neutralization potential on the potentiometric titration curves. Half-neutralization potentials in MeCN of the phenol amine compounds titrated in this study are given in Table 3. LDMHH2 has a more positive half-neutralization potential. This is in contrast to what is expected. LHH2 acts as a stronger base. The hydrogen bond formed between phenolic OH group and the aminic nitrogen is considered to create this effect.
A strong hydrogen bond is known to exist between phenolic OH group and aminic and iminic nitrogen atoms. Due to this hydrogen bond hydrogen can be partially transferred to the nitrogen atom [25–27]. The higher the electron density of the aminic nitrogen atoms, the stronger the phenolic hydrogen will be bonded to this nitrogen. In this case it is not possible to determine the status of the charge density on the nitrogen atoms by potentiometric titration.
Therefore, in order to determine the electron density on the aminic nitrogen atoms, phenolic groups have been transformed into methoxide groups and titrated in MeCN.
Since no hydrogen bonds are formed with methoxy groups electron density of the nitrogen atoms can be determined more clearly.
The titration curves LDMH2 and LDMHH2 obtained in acetonitrile medium are given in Fig. 3a, b as an example. It is seen that 1. half neutralization potentials for bis-N,N′-(2-methoxybenzyl)-1,3-propanediamine −185 mV, bis-N,N′-(2-methoxybenzyl)-2,2′-dimethyl-1,3-propanediamine −235 mV, respectively. These values show that the nitrogen atoms of bis-N,N′-(2-methoxybenzyl)-2,2′-dimethyl-1,3-propanediamine have higher electron density. Due to this higher electron density, the bi-nuclear complex remains somewhat more stable after thermal separation of the DMF molecules from this ligands complex.
This is also seen in bond lengths. Suitable crystals of complexes III and IV have been prepared and their molecular models have been obtained by X-ray diffraction method. Figure 4 shows the PLATON drawing [28] for complexes III (a) and IV (b) and their selected crystallographic and experimental data are given in Table 4, some of the important coordinative bond lengths and angles are tabulated in Tables 5 and 6, respectively.
As it can be seen in Fig. 4a and b Ni(II) ions are in an octahedral and Zn(II) ions in a tetrahedral coordination sphere.
In complex III distances between Ni(II) and the DMF oxygen’s are found to be 2.060 and 2.096 Å. In complex IV these distances are found as 2.109 and 2.140 Å. Bonds in complex IV are clearly longer. This shows that there is less electron requirement for the Ni(II) in complex IV. Since the nitrogen atoms of the ligand in complex IV have higher electron densities the bond between Ni(II) and DMF is longer. Ni(II) ion in complex III needs more electrons, therefore it attracts the electrons of the DMF molecules and the respected bond length is shorter. Therefore, the temperature at which the DMFs leave the structure goes up to 200 °C.
As seen from Fig. 4a and b, in both complexes Ni(II) ion has O4N2 and Zn(II) ion has O2Cl2 coordination sphere. Ni(II) ion is coordinated between two phenolic oxygen and two iminic nitrogen of the organic ligand. These two phenolic oxygen and two iminic nitrogen donors form a basal plane. Ni(II) ions are located approximately at the middle of this basal plane. The oxygen atoms of two DMF molecules coordinate Ni(II) ion up and down of the basal plane. Therefore, Ni(II) ion is in an octahedral coordination sphere. Two DMF oxygen are in apical position. As seen from Table 5 the bond lengths around the Ni(II) ion in complex III and IV vary between 2.060–2.096 and 2.048–2.140 Å. The bond lengths in the basal plane in complex III are 2.079, 2.084, 2.074 and 2.073 Å and they are very close to each other. The bond lengths in apical positions were found to be 2.060 and 2.096 Å in the complex III and they do not differ from each other very much. The bond lengths in the basal plane and apical positions of complex IV were found to be between 2.048–2.072 and 2.109–2.140 Å. These values are also very close to each other. However, the bonds in the basal plane of complex IV are shorter and the bonds at apical positions are considerable longer than those of complex III. This shows that the coordinative bonds in the basal plane of complex IV are more stabile than those in complex III. This may be attributed to the fact that higher electron density of the nitrogen atoms in LDMH2−. Only the coordination sphere of Ni(II) in complex III is close to ideal. Complex IV has a more distorted coordination sphere. The bond angles support this fact. The coordination sphere of Zn(II) ion is a distorted tetrahedral. ZnCl2 molecule forms a μ bridge with phenolic oxygens.
Supplementary materials
Crystallographic data for the structural analysis have been deposited with the Cambridge Crystallographic Data Center, CCDC 687246 for complex III and CCDC 687245 for complex IV. Copies of this information can be obtained free of charge from The Director, CCDC, 12 Union Road, Cambridge, CB2, 1EZ, UK (fax: +44-1223-336-033; e-mail: deposit@ccdc.cam.ac.uk or http://www.ccdc.cam.ac.uk).
References
Gerli A, Hagen KS, Marzilli LG. Nuclearity and formulation of SALPN2- complexes formed from M(O2CCH3)2: resolution of longstanding problems by X-ray crystallography. Inorg Chem. 1991;30:4673–6.
Uhlenbrock S, Wegner R, Krebs B. Syntheses and characterization of novel tri- and hexa-nuclear zinc complexes with biomimetic chelate ligands. JCS Dalton Trans. 1996;3731–6.
Mikuriya M, Ikemi S, Lim JW. Monodentate, didentate, and tridentate ligation of a Schiff-base ligand in a tetranuclear zinc(II) complex. Bull Chem Soc Jpn. 2001;74:99–100.
Shi DH, You ZL, Xu C, Zhang Q, Zhu HL. Synthesis, crystal structure and urease inhibitory activities of Schiff base metal complexes. Inorg Chem Commun. 2007;10:404–6.
Drew MGB, Prasad RN, Sharma RP. Structures of (N,N'-trimethylenedisalicylideneaminato)nickel(II) (1) and (N,N'-trimethylenedisalicylideneaminato)copper(II) (2). Acta Cryst. 1985;C41:1755–8.
Colon ML, Qian SY, Vanderveer D, Bu XR. Chiral bimetallic complexes from chiral salen metal complexes and mercury(II) halides and acetates: the anionic groups interact with Cu(II) in apical position. Inorg Chim Acta. 2004;357:83–8.
Sanmartin Matalobos J, Garcia-Deibe AM, Fondo M, Navarro D, Bermejo MR. A di-μ-phenoxo bridged zinc dimer with unfamiliar spatial arrangement. Inorg Chem Comm. 2004;7:311–4.
Atakol O, Nazır H, Arıcı C, Durmuş S, Svoboda I, Fuess H. Some new Ni-Zn heterodinuclear complexes: square-pyramidal nickel(II) coordination. Inorg Chim Acta. 2003;342:295–300.
Ülkü D, Kaynak FB, Atakol O, Aksu M. Crystal structure of {[μ-bis(salicylidene)-1,3-propanediaminato]-copper(II)} dibromozinc(II). Anal Sci. 2003;19:799–800.
Döring M, Hahn G, Imhof W. Di-μ-bromo-2κ2Br:2'κ2Br-bis[bis(μ-acetylacetonato-1κO:2κO')bromo-2κBr-bis(tetrahydro-furan-1κO)cadmium(II)nickel(II)]. Acta Cryst. 2006;E62:m429–31.
Aminabhavi TM, Biradar NS, Divakar MC. Bimetallic complexes of nickel(II)-tetradentate Schiff bases with tin(IV), selenium(IV) and tellurium(IV) chlorides. Indian J Chem. 1986;25A:283–4.
Ülkü D, Kaynak FB, Atakol O. {[μ-Bis(salicylidene)-1,3-propanediaminato]bis(3,5-dimethylpyridine)nickel(II)} dibromozinc(II). Acta Cryst. 2002;E58:m251–3.
Ercan F, Arıcı C, Akay A, Atakol O, Ülkü D. {[μ-Bis(salicylidene)-1,3-propanediaminato](3-methylpyridine)copper(II)} diiodozinc(II). Acta Cryst. 1999;C55:925–8.
Arıcı C, Aksu M. Crystal structure of {[μ-N,N'-bis(salicylidene)-1,3-propanediaminatodimethylformamide]zinc(II)}diiodozinc(II). Anal Sci. 2002;18:727–8.
Aneetha H, Panneerselvam K, Liao TF, Lu TH, Chung CS. Syntheses, structures, spectra and redox properties of alkoxo- and phenoxo-bridged diiron(III) complexes. JCS Dalton Trans. 1999;16:2689–94.
Arıcı C, Svoboda I, Sarı M, Atakol O, Fuess H. {Bis(N,N-dimethylformamide)[μ-N,N'-bis(salicylidene)-1,3-propanediaminato] nickel(II)}dibromozinc(II). Acta Cryst. 2001;C57:31–2.
Atakol O, Ercan F, Arıcı C, Fuess H, Svoboda I. {[μ-Bis(salicylidene)-1,3-propanediaminato]bis(4-methylpyridine) nickel(II)}dichlorozinc(II). Acta Cryst. 1999;C55:2023–6.
Enraf-Nonius. Cad4 Express Software Version 1.1. Delft, The Netherlands: Enraf-Nonius, 1993.
Sheldrick GM. SHELXS97 and SHELXL97. Programs for crystal structure solution and refinement. University of Gottingen, Germany, 1997.
Farrugia LJ. WinGX Program for Crystallography Package. J Appl Cryst. 1999;32:837.
You ZL, Zhu HL, Liu WS, Anorg Z. Solvolthermal syntheses and crystal structures of three linear trinuclear Schiff base complexes of zinc(II) and cadmium(II). Allg Chem. 2004;630:1617–22.
Freedman HH. Intramolecular hydrogen bonds. I. Spectroscopic study of the hydrogen bond between hydroxyl and nitrogen. J Am Chem Soc. 1961;83:2900–5.
Durmuş S, Ergün Ü, Jaud JC, Emregül KC, Fuess H, Atakol O. Thermal decomposition of some linear trinuclear Schiff base complexes with acetate bridges. J Therm Anal Calorim. 2006;86:337–46.
Huber W. Titrationen in Nichtwasserigen Lösungsmitteln, Band 1, Akademische Verlagsgesellschaft, Frankfurt/Main, 1964, p. 130–58.
Nazır H, Yıldız M, Yılmaz H, Tahir MN, Ülkü D. Intramolecular hydrogen bonding and tautomerism in Schiff Bases. Structure of N-(2-pyridil)-2-oxo-1-naphthylidenemethylamine. J Mol Struct. 2000;524:241–50.
Maciejewska D, Pawlak D, Koleva V. Hydrogen bonding and tautomerism of benzylideneanilines in the solid state. J Phys Org Chem. 1999;12:875–80.
Tatar L, Nazır H, Gümüşer M, Kale C, Atakol O. Synthesis, crystal structure and electrochemical behaviour of water soluble Schiff bases. Z Kristallogr. 2005;220:639–42.
Spek AL. PLATON, Program for crystal molecular drawing. University of Ultrech, The Netherlands, 2000.
Acknowledgements
The authors also thank the Ankara University Scientific Research Fund (Project No: 20070705118) for their support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zeybek, B., Ateş, B.M., Ercan, F. et al. The effect of ligand basicity on the thermal stability of heterodinuclear NiII–ZnII complexes. J Therm Anal Calorim 98, 377–385 (2009). https://doi.org/10.1007/s10973-009-0302-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-009-0302-2