Abstract
The effects of cashew gum over heart samples of spontaneously hypertensive rats (SHR) were studied by Thermal Analysis and Micrographs. During the experiment, ten rats received daily by gavage 500 mg/kg of cashew gum (CG groups) and the other ten rats received only fresh water (Control groups). After 17 weeks of experiment, blood pressure in CG groups was approximately 8% lower and after 24 weeks it was 20% lower than Control groups. At seven months of experiment ten rats (five of each group: Control 7 and CG 7) and at nine months of experiment the other rats (Control 9 and CG 9) were sacrificed. Pieces of left ventricle were studied by thermogravimetry (TG), differential thermal analysis (DTA) and microscopy. The myocardial appearance of CG groups showed the presence of greater number of cardiomyocytes and more capillaries among the cells. The myocardium of Control groups showed more areas with diffuse interstitial fibrosis and hypertrophied cardiomyocytes. TG curves depict two steps for all samples. CG 7 and CG 9 presented the least amount of residues (6.0 ± 0.5) in the heart. DTA curves depict two endothermic events (Tpeak1: 85 °C; Tpeak2: 298 °C). The results suggest that cashew gum contributes to improve cardiovascular health of SHR and to postpone the aging process of their hearts.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cashew gum (CG) is a tree exudate from Anacardium occidentale L., widely known for its nuts, which is used as a food ingredient. It is composed of complex branched heteropolysaccharides and their calcium, magnesium, potassium and sodium salts, with a weight-average molecular weight, M w ~ 1.5 × 104. Cashew gum interacts with water and it has emulsifier, adhesive and stabilizer properties [1]. It can be considered a functional dietetic fiber [2]. Cardiovascular diseases are the major cause of death in the world and are influenced by genetic, environmental factors and aging [3]. In 2003, heart diseases were responsible for 32% of the total number of deaths among Brazilian people and they were the cause of death to 950 thousand people in the USA [4]. Hypertension contributes to increase cardiovascular problems and accelerates the aging process of the heart: significant loss of cardiomyocytes and hypertrophy of remaining cells. By the way, dietetic fibers are used as cardiovascular system promoter [2]. Additionally, studies have demonstrated that Thermal Analysis is an interesting tool in the evaluations of organs and blood characteristics [4–6]. The present work study aims to study, by Thermal Analysis, the functional effect of cashew gum in the heart samples of spontaneously hypertensive rats (SHR).
Experimental
Animals
Twenty male spontaneously hypertensive rats (SHR) were used in this study. From 3 to 9 months old they were maintained in the State University of Rio de Janeiro. The University Standing Committee on Animal Research had approved the protocols, which conforms to the ‘Guide for the Care Use of Laboratory Animals’ published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1985).
The rats were kept in polypropylene cages, in a controlled temperature (21 °C), and submitted to 12 h light/dark and air extrusion. At the beginning of the experiment (3 months old) animals were shared in two different groups (ten animals each) which received daily by gavage: (1) 500 mg/kg of cashew gum (CG group) and (2) only fresh water (Control group). All animals were weekly weighed and the systolic blood pressure (BP) was verified by non-invasive method of tail-cuff plethysmography in conscious rats.
At seven months of experiment ten rats (five of each group: Control 7 and CG 7) were sacrificed after anaesthesia with intraperitoneal sodium pentobarbital and their vascular system were perfused and fixed with formaldehyde solution. At nine months of experiment the other rats (Control 9 and CG 9) were sacrificed. Small pieces of left ventricle from animals were studied by Thermal analysis and micrographs.
Thermal analysis (TG-DTA experiments)
The thermal analyses have been performed on a TA 2960 simultaneous TG-DTA model (TA Instruments, USA). Two pieces of left ventricle heart (10–12 mg) of each SHR (total of ten samples per group) were heated from 30 to 800 °C, at a 10 K/min heating and nitrogen atmosphere; and an average was obtained for each group.
Statistical analysis
Differences between groups were tested with one-way ANOVA test (GrapPad Prism 4.01, San Diego, USA) with the significant level of p = 0.05.
Micrographs
The left ventricle (LV) fragments were embedded in Paraplast plus (Sigma-Aldrich Chemical Co., St. Louis, USA), sectioned at 2 and 10 μm thickness, and sections were stained with hematoxylin-eosin and trichrome methods (Masson and picro sirius red stains). Light microscopy was performed using a microscope Leica DMRBE (Wetzlar, Germany), a Kappa video camera (Gleichen, Germany) and a Sony Trinitron monitor (Pencoed, UK).
Results and discussion
Systolic blood pressure
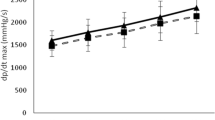
Rats from Control groups showed systolic (BP) blood pressure around 200 ± 5 mmHg and Cashew Gum groups showed BP from 200 ± 5 mmHg to around 160 ± 8 mmHg (Fig. 1). Significant differences could be observed after 14 weeks of experiment. After 22 weeks of experiment, BP in Cashew group was approximately 20% lower than in the Control group.
Thermal analysis
Figure 2 shows TG/DTG and DTA curves of heart samples of Cashew Gum 7 (CG 7) group. The curves depict two loss mass stages, which agree with anterior thermal studies of SHR hearts [4, 6]. The first mass loss stage of the CG 7 group occurs from 52 °C to 97 °C with 49.6 ± 1.9% of mass loss and the second one occurs from 283 °C to 341 °C with 9.2 ± 1.2%, these stages are likely related to water and blood serum loss, and the decomposition of the substances like proteins, lipids and others, respectively. The residue at 800 °C is 6.9 ± 0.4%, probably due to inorganic substances because, usually, organic substances degrade at much lower temperatures. DTG curve shows two mass degradation stages around 80 and 220 °C and multiple stages from 265 °C to 380 °C. DTA peaks shows three principal endothermic events (T peak1: 84 °C; T peak2: 256 °C;T peak3: 301 °C). The first event (T peak1: 84 °C) is probably related to the thermal denaturation of proteins compounds, such as myosin and actin; as reported by Mothe et al. [4], who showed two endothermic events related to heart denaturation of proteins at 67 °C and 76 °C. The third event (T peak3: 301 °C) suggests the presence of lipids and degradation of fat.
Figure 3 shows TG/DTG and DTA curves of heart samples of Control 7 group. TG curve presents two mass loss stages. The first and main mass loss stage starts at 52 °C to around 97 °C causing 49.6 ± 1.9% of mass loss. The second stage occurs from 283 °C to 341 °C with 9.2 ± 1.2% of mass loss. The residue quantity 800 °C is 6.9 ± 0.4%, and may indicate the presence of inorganic carbon and metals as iron, calcium, magnesium, potassium and sodium. DTG curve presents two stages of decomposition from 80 °C to 220 °C and multiple stages from 265 °C to 380 °C. DTA curve indicates the presence of endothermic events during all temperature range investigated (T peak1: 84 °C; T peak2: 256 °C; T peak3: 301 °C). These peaks also indicate denaturation of proteins, degradation of proteins and degradation of fat, respectively.
TG curve of heart samples of CG9 also shows only two loss mass stages (Fig. 4). The first stage occurs from 48 to 101 °C with 51.5 ± 2.5% of mass loss. In the second stage occur multiple stages from 280 °C to 341 °C. At 800 °C the samples presents 6.0 ± 0.5% of residues. DTG peak shows two loss mass stages. DTA curve indicates endothermic events during the whole investigated temperature range (T peak1: 86 °C; T peak2: 298 °C).
TG/DTG and DTA curves of Control 9 heart sample are presented in Fig. 5. TG curve of SHR heart sample shows two mass loss stages. The residue at 800 °C is 6.7 ± 0.3%, likely related to the presence of metals as iron, calcium, magnesium, potassium and sodium. DTG curve also shows two mass degradation stages around 80 °C and 240 °C and multiple stages from 265 °C to 450 °C. DTA curve indicates endothermic processes during the whole investigated temperature range (T peak1: 85 °C; T peak2: 298 °C).
Among all groups, CG 9 presented the least amount of residues (6.0 ± 0.5), probably related to the best quality of its heart tissue (Fig. 6).
Micrographs
Cardiomyocytes loss and the consequent decreasing of the functional reserve of the heart are a natural phenomenon during the aging of mammals, mainly in the left ventricular myocardium. The arterial hypertension accelerates this degradation process.
The myocardial appearance of cashew groups (Fig. 7b and d) shows the presence of greater number of cardiomyocytes and more capillaries among the cells, that suggest better myocardial perfusion preservation in these animals than in the control groups (Fig. 7a and c). The myocardium of the control groups shows more areas with diffuse interstitial fibrosis and hypertrophied cardiomyocytes, suggesting that cashew gum plays an important role in the heart cells preservation as reducer of blood pressure. In the cashew gum groups (with lower BP) there is greater number of cardiomyocytes, desired effect for improvement of cardiovascular system.
The cardiomyocytes loss is attenuated, possibly, by cashew gum ingested; which modified the natural tendency of SHR. This is a promissory result because the goal is to postpone the consequences of the cardiomyocyte number decrease and heart failure in hypertension.
Conclusions
Thermal analysis seems to be an interesting tool in the thermal study of functional effects of fibers over cardiovascular system. TG curves showed two steps for all samples. Less residues remained in the TG analysis of CG 9 (6.0 ± 0.5%) and CG 7 (6.3 ± 0.5%) than Control 9 (6.7 ± 0.3%) and Control 7 (6.9 ± 0.5) heart. DTA curves depict two endothermic events (T peak1: 85 °C; T peak2: 298 °C). The cardiomyocytes loss caused by aging process was, possibly, attenuated by cashew gum ingested, which modified the BP increase and LV cardiomyocytes decrease natural tendency of SHR. The TG/DTG and DTA results showed that cashew gum may have contributed for improvement of cardiovascular health of SHR, however even more extended research must be done to obtain sufficient evidence on this matter.
References
Mothé CG, Rao MA. Thermal behavior of gum arabic in comparison with cashew gum. Thermochim Acta. 2000;357–358:9–13.
Pimentel CVMB, Francki VM, Gollücke APB. Alimentos Funcionais–Introdução às principais substâncias bioativas em alimentos. 1st ed. São Paulo: Varela editora; 2005.
Aguila MB, Pinheiro AR, Mandarim-de-Lacerda CA. Spontaneously hypertensive rats left ventricular cardiomyocyte loss attenuation through different edible oils long-term intake. Int J Cardiol. 2005;100(3):461–6.
Mothé CG, Carestiato T, Aguila MB, Mandarim-de-Lacerda CA. Thermal behavior of the heart of SHR and Wistar rats. J Therm Anal Cal. 2005;80:429–33.
Mothé CG, Carestiato T, Aguila MB, Mandarim-de-Lacerda CA. Thermoanalytical investigation of blood. J Therm Anal Cal. 2006;85:247–51.
Mothé CG, Carestiato T, Aguila MB, Mandarim-de-Lacerda CA, Mandarim-de-Lacerda CA. Thermoanalytical study of organs of spontaneously hypertensive rats. J Therm Anal Cal. 2006;85:61–3.
Acknowledgements
Authors thank to agencies Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for their financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Carestiato, T., Aguila, M.B. & Mothé, C.G. The effects of cashew gum as anti-hypertensive agent. J Therm Anal Calorim 97, 717–720 (2009). https://doi.org/10.1007/s10973-009-0283-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-009-0283-1