Abstract
Hydrotalcite was synthesised by co-precipitation method, calcined and characterized by XRD, BET, IR and TG/DTA/DTG analyses and tested as solid base catalyst in the transesterification of soybean oil with methanol, achieving a methyl ester content of 99.5%. The thermal decomposition of hydrotalcite calcined occurred in four mass loss steps at 28, 105, 203 and 400 °C. The hydrotalcite was recovered and through a simple evaluation by TG/DTA/DTG techniques it was found that at 500 °C is the temperature, where the organic matter should be eliminated from the catalyst. This study shows the importance of thermal analysis in the evaluation of the recovery temperature of hydrotalcite.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hydrotalcites, Mg6Al2(OH)16CO3·4H2O, are layered double hydroxides (LDHs) and are substantially anionic clays [1–3]. The structure of hydrotalcite resembles that of brucite, Mg(OH)2, where the magnesium cations are octahedrally coordinated by hydroxyl ions, resulting in stacks of edge-shared layers of the octahedral. In the hydrotalcite structure, part of the Mg2+ ions are replaced by Al3+ ions forming positively charged layers. This substitution is compensated by interlayer anions (usually CO3 2−), which are situated between the brucite-like layers [4–8].
Biodiesel can be produced by the transesterification of triglycerides of vegetable oils using hydrotalcite as an alkaline heterogeneous catalyst. The process presents several advantages such as: facile separation of the final products (biodiesel and glycerol) and the possible reutilization of the catalyst, reducing environmental drawbacks [9, 10]. Moreover, there are some economic advantages from the use of solid catalysts because its can be potentially used for long periods of time, improving the economics of biodiesel production [11].
In the present work, hydrotalcite was synthesised by co-precipitation method, calcined and characterized by TG/DTA/DTG, XRD, BET and IR analyses. The catalytic properties of the material were investigated in the methanolysis of commercial soybean oil and the composition of the obtained methyl ester phase was determined by gas chromatography (GC). The thermal stability and the thermal decomposition of the poisoned hydrotalcite were investigated by TG/DTA/DTG. The physico-chemical properties of regenerated hydrotalcite were studied by XRD, BET and IR techniques.
Experimental
Materials
The chemicals were: Mg(NO3)2·6H2O, Al(NO3)3·9H2O, NaOH, Na2CO3 and methanol (MeOH). All the reactants were purchased from Vetec. The soybean oil was purchased from a local grocery store.
Synthesis
Hydrotalcite, with an Al/(Al + Mg) theoretical atomic rate of ~0.25, was synthesized according to [12]. Solution A (51.9 g Mg(NO3)2·6H2O, 25.1 g Al(NO3)3·9H2O and 90 mL distilled water) was added to solution B (21.8 g NaOH, 3.5 g Na2CO3 and 90 mL of distilled water) at a rate of 1 mL min−1. The mixture was vigorously stirred for an hour at room temperature. Then, it was aged for 18 h, at 65 °C, without stirring. The material thus obtained (LDHs) was filtered, washed to eliminate alkali metal and nitrate ions, and dried at 65 °C for 18 h. Finally, the obtained product was calcined at 200 °C in air for 18 h. This sample was labeled as LDHc.
Catalytic reaction
The catalytic activity of LDHc was tested in the transesterification of commercial soybean oil with methanol using in a Parr 4843 reactor. LDHc (5 mass% of the oil mass) was mixed with appropriated amounts of methanol and oil (molar ratio oil:MeOH was 1–12). The reaction was carried out at 180 °C for 2 h and the obtained product was separated from the poisoned catalyst (labelled LDHp) by filtration.
Characterization
The XRD measurements were performed on a PW 3710 BASED powder X-ray diffractometer using Cu Kα, over a 2θ range of 5–80°. The phases were identified using the powder diffraction file (pdf) database (JCPDS, International Centre for Diffraction Date). Surface area and pore diameter were obtained using N2 adsorption–desorption (BET/BJH) in a Quantachrome, model NOVA 1200. Prior to N2 adsorption, the samples were outgassed for 2 h at 200 °C. IR spectra of samples were investigated in the range 4000–450 cm−1. Spectra were recorded on a Thermo Electron Corporation, model IR 100. Thermal decomposition and thermal stability of the hydrotalcites were evaluated by thermogravimetric analysis (TG/DTG) and differential thermal analysis (DTA) carried out on a Shimadzu instrument, model DTG-60H, under a flow of air at a 10 °C min−1 heating rate up to 1000 °C. The composition of the obtained methyl ester phase was determined by gas chromatography (GC).
Results and discussion
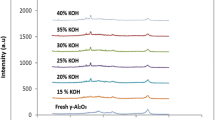
The XRD patterns of all the studied hydrotalcites are shown in Fig. 1. The corresponding X-ray diffractograms are consistent with those expected for hydrotalcite. The XRD revealed that hydrotalcite is monophase. However, during calcination of LDHp at 500 °C for 18 h occurred formation of mixed Mg–Al oxides phases. This fact was confirmed by the XRD patterns of the regenerated hydrotalcite (LDHr).
The surface areas of LDHs and LDHc, calculated according to Brunauer–Emmett–Teller (BET) method were 134 and 102 m2g−1, respectively. The pore diameter, calculated by BJH method, increase from 33.9 Å for LDHs to 34.1 Å for LDHc. These results suggest that LDHc could have a high catalytic activity.
The IR spectra of the hydrotalcites are presented in Fig. 2. All the samples exhibited a band at approximately 3455 cm−1 that was ascribed to the νOH stretching vibration of the hydroxyl groups attached to Al and Mg and to the O–H stretching vibration of surface and interlayer water molecules. The LDHc showed absorption bands at around 1463 cm−1 and 1345 cm−1 corresponds to the co-intercalation of CO3 2− [13]. The absorption bands below 1000 cm−1 are generally assigned to the metal-oxygen skeletal stretching and bending vibrations of the hydrotalcite. The band centered at 734 cm−1 is attributed to M–O (M–O–M or O–M–O) bending deformation vibrations in the brucite-like sheets [14, 15].
IR results showed that LDHp (Fig. 2) has absorption bands at around 2923 cm−1, 2853 cm−1 and 1742 cm−1 that were assigned to organic matter adsorbed in the catalyst. The increasing in the intensity of νOH stretching was also assigned the organic matter adsorbed in LDHp. The absence these bands in the IR spectra of LDHr indicated that the organic matter was eliminated by calcination at 500 °C. Furthermore, the absorption band at approximately 1369 cm−1, indicated that carbonates groups were still present after calcination [13].
The thermal behaviour of LDHc is shown in Fig. 3. Four mass loss steps were observed. The first one (between 28–105 °C) was attributed to the loss of adsorbed water. The second mass loss, between 105 and 203 °C, corresponded to the elimination of interlayer water, without collapse of the hydrotalcite structure. The third (at 203 °C) and the fourth (at 400 °C) mass losses were attributed to the decomposition of interlayer carbonate anion present in the brucite-like layers and the dehydroxilation of OH groups in the hydrotalcite [16, 17].
The thermal analysis of the LDHp (Fig. 4) showed the same LDHc’s mass losses and more three consecutive mass losses (from 265 to 430 °C), noticed by the DTG curves, which are related to the decomposition of organic matter. Through thermal analyses it was found that the temperature of recovery of the LDHp was 500 °C.
LDHc showed high activity for base-catalized transesterification of soybean oil, achieving a methyl ester content of 99.5%. The activity of the LDHr was checked in an experiment carried out at the same conditions of LDHc. The reaction also exhibited a good catalyst performance, yielding about 71% of methyl ester.
Conclusions
In the base-catalized transesterification of soybean with methanol, high activity was achieved using hydrotalcite. Thermal analysis was successfully used to study the thermal decomposition and to determine the appropriate recovery temperature of hydrotalcite. The catalyst showed four steps of thermal transformation that were related to evaporation of adsorbed water (28 °C), elimination of the interlayer structural water (105 °C), decarbonation (203 °C) and dehydroxylation (400 °C). It was found that the poisoned hydrotalcite could be recovered by calcination at 500 °C and that it could be reused as catalyst in the transesterification of soybean oil. This study showed as thermal analysis has proven most useful for characterization of solid catalysts.
References
Frost RL, Musumeci AW, Kloprogge JT, Weier ML, Adebajo MO, Martens WN. Thermal decomposition of hydrotalcite with hexacyanoferrate (II) and hexacyanoferrate (III) anions in the interlayer. J Therm Anal Cal. 2006;86:1–209.
Caoani F, Trifiro F, Vaccari A. Hydrotalcite-type anionic clays: preparation, properties and applications. Catal Today. 1991;11:173–301.
Palmer SJ, Spratt HJ, Frost RL. Thermal decomposition of hydrotalcite with variable cationic ratios. J Therm Anal Cal. 2009;95(1):123–9.
Bouzaid JM, Frost RL, Martens WN. Thermal decomposition of the composite hydrotalcites of iowaite and woodallite. J Therm Anal Cal. 2007;89:511–9.
Palmer SJ, Frost RL, Nguyen T. Thermal decomposition of hydrotalcite with molybdate and vanadate anions in the interlayer. J Therm Anal Cal. 2008;92:879–86.
Kloprogge JT, Hickey L, Frost RL. The effect of varying synthesis conditions on zinc chromium hydrotalcite: a spectroscopic study. Mater Chem Phys. 2005;89:99–109.
Frost RL, Weier ML, Kloprogge JT. Raman spectroscopy of some natural hydrotalcites with sulphate and carbonate in the interlayer. J Raman Spectrosc. 2003;34:760–8.
Bouzaid JM, Frost RL, Musumeci AW, Martens WN. Thermal decomposition of the synthetic hydrotalcite woodallite. J Therm Anal Cal. 2006;86:745–49.
Cantrell DG, Gillie LJ, Lee AF, Wilson K. Structure-reactivity correlations in MgAl hydrotalcite catalysts for biodiesel synthesis. Appl Catal A. 2005;287:183–90.
Di Serio M, Ledda M, Cozzolino M, Minutillo G, Tesser R, Santacesaria E. Transesterification of soybean oil to biodiesel by using heterogeneous basic catalysts. Ind Eng Chem Res. 2006;45:3009–14.
Lotero E, Liu Y, Lopez DE, Suwannakarn K, Bruce DA, Goodwin JG Jr. Synthesis of biodiesel via acid catalysis. Ind Eng Chem Res. 2005;44:5353–63.
Reichle WT. Catalytic reactions by thermally activated, synthetic, anionic clay minerals. J Catal. 1985;94:547–57.
Mustrowski P, Chmielarz L, Bozek E, Sawalha M, Roessner F. Acidity and basicity of hydrotalcite derived mixed Mg-Al oxides studied by test reaction of MBOH conversion and temperature programmed desorption of NH3 and CO2 Mater Res Bull. 2004;39:263–81.
Millange F, Walton RI, Lei LX, O’Hare D. Efficient separation of terephthalate and phthalate anions by selective ion-exchange intercalation in the layered double hydroxide Ca2Al(OH)6.NO3.2H2O. Chem Mater. 2000;12:1990–4.
Frunza M, Lisa G, Popa MI, Miron ND, Nistor DI. Thermogravimetric analysis of layered double hydroxides with chloramphenicol and salicylate in the interlayer space. J Therm Anal Cal. 2008;93:373–9.
Wie W, Peng H, Chen L. Calcined Mg-Al hydrotalcites as solid base catalysts for methanolysis of soybean oil. J Mol Catal A. 2006;246:24–32.
Kameda T, Yoshioka T, Watanabe K, Uchida M, Okuwaki A. Dehydrochlorination behavior of a chloride ion-intercalated hydrotalcite-like compound during thermal decomposition. Appl Clay Sci. 2007;35:173–9.
Acknowledgements
The authors thank UFPA, CAPES, CNPq and FAPESPA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Queiroz, R.M., Pires, L.H.O., de Souza, R.C.P. et al. Thermal characterization of hydrotalcite used in the transesterification of soybean oil. J Therm Anal Calorim 97, 163–166 (2009). https://doi.org/10.1007/s10973-009-0246-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-009-0246-6