Abstract
Several new complexes of Schiff bases ligands H4La and H4Lb with transition metal ions such as Cr(III), Fe(III), Co(II) and Zn(II) are synthesized. Elemental analysis, infrared, UV–Vis and thermal analysis, as well as conductivity and magnetic susceptibility measurements are used to elucidate the structure of the newly prepared metal complexes. A square planar geometry is suggested for Zn(II) complexes, while an octahedral geometry suggested for the Cr(III), Fe(III) and Co(II) complexes. The thermal decomposition of complexes was found to be first order reaction and the thermodynamic parameters corresponding to the different decomposition steps were reported.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The transition metal complexes with Schiff bases derived from aliphatic amines and salicylaldehyde drew the interest of many workers based on their similarity to biological oxygen carrier [1–3] and their ability to insert oxygen into organic substances [4, 5]. Some Schiff bases complexes show catalytic properties [6] or act as oxygen carriers [7] and antifungal agents; the activity increases by the presence of hydroxyl groups in the ligands [8–12]. The interaction of DNA with complexes [Cr(Schiff base) (H2O)2] · ClO4 [13, 14] was reported.
The thermal decomposition of the complexes of Cr and Mo with salicylidene aniline derivatives was found to be first order reaction and the thermodynamic parameters corresponding to different decomposition steps were reported [15]. Many authors determined the thermodynamic parameters of metal complexes from the TG data [16–18]. Recently, the thermal decomposition pathways of some transition metal complexes are reported [19, 20]. Despite the fact that Schiff base complexes were the subject of several interesting studies, yet the chromone Schiff bases were included in few studies [21–24]. This work aims to synthesize and characterize new Cr(III), Fe(III), Co(II) and Zn(II) complexes of Schiff base ligands, H4La and H4Lb derived from the condensation of 6-formyl-5,7-dihydroxy-2-methylbenzopyran-4-one with ethylenediamine or trimethylenediamine (Scheme 1). The complexes are characterized by elemental analysis, IR, UV–Visible, 1H-NMR spectroscopy, and studied using thermal analysis. The thermal stability and the thermodynamic parameters of the different decomposition steps of the complexes are calculated.
Experimental
The chloride salts of Cr(III), Fe(III), Co(II) and acetate salts of Co(II), Zn(II) and perchlorate salts of Co(II), potassium dichromate, ethylenediamine, trimethylenediamine, visnagine are Aldrich Chemicals. The organic solvents are reagent grade chemicals and used as received.
Analysis and physical measurements
Carbon, hydrogen, nitrogen and chlorine were analyzed by standard microanalysis methods at Microanalytical center, Cairo University, Giza, Egypt. IR spectra of the ligands and their metal complexes, as KBr discs, were recorded on a Shimadzu FTIR spectrometer. 1H-NMR spectra of the ligands and their Zn(II) complexes, in DMSO-d6, were recorded on Varian 300 MHz NMR spectrometer at room temperature using TMS as an internal standard. UV–Vis spectra of the metal complexes in DMF were recorded on UV–Vis-NIR 3101 PC Shimadzu spectrophotometer.
Magnetic susceptibilities of the complexes were measured at room temperature using Cambridge, England Sherwood Scientific magnetic susceptibility balance. The effective magnetic moments were calculated from the expression:
where X M is the molar susceptibility corrected using Parcel’s constants for diamagnetism of all atoms in the compounds [25].
Molar conductivity of 10−3 M solutions of the complexes in DMF was measured on the conductivity meter ORION model 150 of 0.6 Pascal’s constant.
Thermal analyses have been carried out using Shimadzu-50 Thermal Analyzer from room temperature to 600 °C with heating rate of 10 °C min−1.
Synthesis of the Schiff-bases H4La and H4Lb
The ligands H4La and H4Lb were prepared in two steps. The first step involved the oxidation of visnagine C13H10O4 (2.40 g, 10.4 mmol) with chromic acid (60 mL of 10% H2SO4 and 40 mL of 10% K2Cr2O7) at 70–80 °C with constant stirring [26]. The product (1 g, 4.27 mmol) was hydrolyzed by refluxing with 25 mL 1:1 HCl to produce 6-formyl-5,7-dihydroxy-2-methylbenzopyran-4-one. The second step involved the addition of a solution of ethylenediamine (0.30 g, 5 mmol) or trimethylenediamine (0.37 g, 5 mmol) to 6-formyl-5,7-dihydroxy-2-methylbenzopyran-4-one (2.20 g, 10 mmol) according to a method described previously [27] (Scheme 1).
Synthesis of the metal complexes
A hot ethanolic solution of the metal salt (10 mL) was gradually added to (20 mL) solution of the ligand in 1:1 molar ratio and the solution was stirred on a water bath for 2 h during which the metal complex precipitated. If the complex did not precipitate, ammonia solution was added dropwisely to adjust the pH value in the range 6–7. The resulting precipitates were filtered off, washed with water, ethanol then diethyl ether, and finally air-dried. The complexes are air stable in the solid state and soluble in DMF or DMSO. The obtained complexes were subjected to CHN elemental analysis at the Micro Analytical center, Faculty of Science, Cairo University. The metal content was determined by recommended methods [28].
Determination of reaction order n of decomposition
We used the Horowitz and Metzger [29] equation, C s = n 1/1−n which is given by:
Where C s is the mass fraction of the complex present at DTG peak temperature T s, W s is the mass remaining at T s, W o and W f are the initial and final masses respectively.
Coats-Redfern method [30]
For a first order process, the Coats-Redfern equation is written after approximation in the form:
where W f is the mass loss at the completion of the reaction, W r is the remaining mass at temperature T, R is the gas constant, E* is the activation energy, θ is the heating rate. Plots of the left-hand side of the last equation versus 1/T give straight lines, where E* and A (Arrhenius constant) are calculated from the slope and the intercept, respectively.
The activation entropy ΔS*, activation enthalpy ΔH* and free energy of activation ΔG* were calculated using the following equations:
where k and h are the Boltzman and Planck constants, respectively.
Results and discussion
The formulae of the complexes, together with the physical properties, elemental analyses and formula masses are listed in Table 1. The isolated solid complexes are stable at room temperature (25 °C), non-hygroscopic and almost insoluble in water and most organic solvents but are soluble in DMF and DMSO. Most of the complexes decomposed on heating at >300 °C.
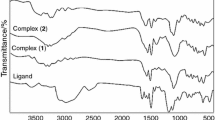
IR spectra
The most important infrared bands of the ligands and the metal complexes with their probable assignments are given in Table 2. The IR spectra of the ligands (H4La and H4Lb) exhibit bands at 3429–3433, 1110–1113, 1222 cm−1 attributed to νOH, δOH def. and δC–OH, respectively. The C=O group in position four shows stretching band at 1658 and 1651 cm−1 for ligands H4La and H4Lb. The azomethine νC=N band overlaps with νC=C as a strong band at 1620 for H4La and 1612 for H4Lb.
The existence of numerous coordination sites in the ligands gives variable bonding modes; however comparison of the IR spectra of the ligands and their complexes reveal that the ligands are bonded to metal ions in one coordination mode. The ligands are quadridentate dibasic (N2O2) donors in the complexes. The spectra proved that the C=N and C7–OH groups are the coordination centers. Strong evidence is (i) The band assigned to the stretching vibration of the azomethine groups of the ligands at (1620, 1612 cm−1) is shifted to lower frequency or higher frequency in metal complexes suggesting that one of the coordination sites of the ligand to the central metal atom is through nitrogen atom of azomethine groups. (ii) The existence of stretching C=O in position 4 which is more or less invariant; indicates that this group is not taking part in coordination. (iii) The band at 1218–1222 cm−1 is ascribed to the stretching vibration of the phenolic υC–O [31], this band is shifted to higher frequency (1222–1272 cm−1) in the IR spectra of the metal complexes indicating that the metal ions are coordinated through the oxygen atoms of the phenolic groups after deprotonation. (iv) The new band in the spectra of all complexes in the frequency range 408–466 and 509–586 cm−1 can be assigned to the stretching frequencies υM–N and υM–O, respectively, which are absent in the IR spectra of the free ligands [32]. The presence of the bands at 1097 and 624 cm−1 in the IR spectrum of Co(II) perchlorate complex with H4Lb are indicative of the presence of non-coordinated perchlorate ion [34].
Proton nuclear magnetic resonance (1H-NMR) spectra
The 1H-NMR spectra of ligand recorded in d6-DMSO show that the phenolic OH proton signals appear at 14.35 and 14.43 ppm (s, 4H) for H4La and H4Lb, respectively, this may be due to that the molecule is planar [33]. In the 1H-NMR spectra of Zn(II) complexes no signals are observed at 14.00–14.50 region, meanwhile a new signal appeared at 7.95 ppm (s, 2H) equivalent to two hydrogens. This signal indicates that two phenolic protons are participated in complex formation and the other two protons are shifted to high field after chelation.
Electronic spectra, magnetic moment studies and molar conductivity
The electronic spectra of both ligands showed a band at 402 nm (24876 cm−1) assigned to n–π* transition, which is shifted to lower wave number on coordination, as a result of the participation of the two azomethine nitrogens in coordination (Table 3).
The electronic spectra of the 1:1 (M:L) Cr(III) complexes display charge-transfer band at 25000 and 27027 cm−1 for H4La and H4Lb, attributed to ligand-to-metal charge-transfer bands. These charge-transfer transitions probably occur from π-orbitals of the Schiff base to the d-orbitals of chromium. The magnetic moment values are 3.85 and 3.65 B.M. indicating the presence of three unpaired electrons (3d3) in Cr(III) ion. These values of magnetic moments are consistent with the octahedral geometry [35, 36].
The visible spectra of Fe(III) complexes show two bands for each complex. The first band occurred at 34247 and 34014 cm−1 for H4La and H4Lb complexes. These bands may be attributed to LMCT transition. The second band at 21142 and 20202 cm−1 for these complexes is due to ligand field (d–d) transitions, this band can be assigned to the transition 2T2g − 2T1g [37]. The magnetic moment values are 5.80 and 5.85 B.M. for these complexes. The calculated μeff value 5.91 B.M. of the magnetic moments are evidence for the octahedral geometry for these chelates [38].
The visible spectra of Schiff bases complexes with cobalt (II) acetate, chloride and perchlorate with ligands H4La and H4Lb are studied. The spectra of the complexes with H4Lb show band in the range of 26525–26954 cm−1 that may be due to a LMCT transition, whereas the complexes with H4La show band in the range 20000–20408 cm−1 assigned to d–d transition and may be attributed to 4T1g → 4T1g(p) [37] transition. The magnetic moments of these cobalt complexes are in the range 4.05–5.01 B.M. which are in good agreement with (3d7) high spin octahedral geometry [37]. On the other hand, the diamagnetic nature of cobalt-H4Lb complex (prepared from Co(II) perchlorate) is taken as a good evidence for oxidation of Co(II) to Co (III) [39].
The electronic spectra of metal chelated of Zn(II) exhibit bands at 27397–27548 cm−1.
The molar conductance value of H4La and H4Lb complexes in DMF (10−3 M) range from 5.0-58.8 ohm−1 cm2 mol−1 suggested non electrolytic nature of these complexes [40, 41]. These values indicate that no anions (OH− and Cl−) are present outside the coordination sphere. The high observed value of cobalt perchlorate complex with H4Lb 149 ohm−1 cm2 mol−1 indicates that this complex is 1:1 ionic compound [41].
TG analysis of solid complexes
For elucidating the molecular structure and thermal stability of the prepared complexes, the TG study is proved successful [42, 43]. The TG analysis was measured for six solid complexes of H4La and H4Lb with Cr(III), Fe(III) and Co(II) ions.
A typical TG plot for the Schiff base H4Lb with Co(II) is shown in Fig. 1.
The initial mass loss up to 200 °C corresponds to the elimination of the hydrated H2O from the complex. Then an inflection is shown indicating the beginning of removal of the more strongly bound (coordinated) H2O molecules which occupy some positions in the coordination sphere. Further heating of the anhydrous complex [ML] results in a continuous mass-loss till a constant mass where the MO or M2O3 residue is formed as a final product. The following mechanism can be proposed for the thermal decomposition of these Schiff base complexes.
-
(a)
For divalent Co(II) complex
\( \begin{array}{lll}{[{\text {CoL}({\text {H}}_{2}{\text {O}})_{2}}]} \cdot 4{\text {H}}_{2}{\text {O}} & {\mathop \rightarrow \limits^{25-200{}^{\,\circ} {\text {C}}}} & {[{\text {CoL}({\text H_{2}{\text {O}}})_{2}}]} \\ {[{\text {CoL}({\text H_{2}{\text {O}}})_{2}}]} & {\mathop\rightarrow\limits^{200 -300^{\,\circ} {\text {C}}}} & {[{\text {CoL}}] } \\ {[{\text {CoL}}]} & {\mathop\rightarrow\limits^{300-720^{\,\circ} {\text {C}}}} & {\text {Intermediate}} \\ {\text {Intermediate}} & {\mathop\rightarrow\limits^{800^{\,\circ} {\text {C}}}} & {\text {CoO}} \end{array}\)
-
(b)
For trivalent M(III) complexes.
\( \begin{array} {lll} {[{\text {ML}}{({\text {H}}_{2}{\text {O}})_{\text n}}] } \cdot {\text {mH}}_{2}{\text {O}} & {\mathop\rightarrow\limits^{25-200^{\,\circ} {\text {C}}}} & {[{\text {ML}}{({\text {H}}_{2}{\text {O}})_{\text n}}]}\\ {[{\text {ML}}{({\text {H}}_{2}{\text {O}})_{\text n}}]} & {\mathop\rightarrow\limits^{200 -300^{\,\circ} {\text {C}}}} & {[{\text {ML}}]} \\ {[{\text {ML}}]} & {\mathop\rightarrow\limits^{300-700^{\,\circ} {\text {C}}}} & {\text {Intermediate}}\\ {\text {Intermediate}} & {\mathop\rightarrow\limits^{800^{\,\circ} {\text {C}}}} & {\text {M}_{2}\text {O}_{3}} \end{array}\)
[CrH2La(OH)(H2O)] · 3H2O complex
The TG plot of the complex displayed several decomposition steps. The first two steps were due to elimination of both lattice and coordinated water molecules. The decomposition of the anhydrous complex starts at temperature range 300–420 °C with mass loss of 41.43%, this decomposition follows a first order kinetic and the E* was calculated (Table 5). The mass loss in the temperature range 420–480 °C was 34.55% due to complete decomposition of the organic part, the Coats-Redfern relation was not verified and E* can not be calculated. The final product was found to confirm the formation of metal oxide residue Cr2O3.
[FeH2La(OH)(H2O)] · 3H2O complex
The anhydrous iron complex [FeH2La(OH)] decomposes in two main steps. The first decomposition step occurs in the temperature range 300–600 °C with a mass loss of 54.57% while the second step exists over temperature range 600–790 °C with a mass loss of 20.42% corresponding to the elimination of the rest of the Schiff base molecule with the formation of Fe2O3 as a metallic residue (13.14%).
[CoH2La(H2O)2] · 4H2O complex
For the anhydrous complex [CoH2La], two decomposition steps are considered. The first step occurs over temperature range 300–480 °C and the second step exists in the range 480–720 °C. Both successive steps produce intermediates which decompose by further heating to give metal residue CoO as a final product.
[CrH2LbCl(H2O)] · 3H2O complex
The TG plot of the above hydrated complex displays four steps. The first two steps occur due to removal of hydrated 3H2O and coordinated H2O molecules in the range 25–200 and 200–300 °C. The next two decomposition steps occurring at 300–645 °C and at 645–750 °C with mass losses 41.11% and 35.62% represent the decomposition of the anhydrous complex to intermediate and to oxide.
[FeH2Lb(OH)(H2O)] · 5H2O
The Fe(III) complex [FeH2Lb(OH)(H2O)] · 5H2O decomposes in three main steps. Two steps of dehydration occur in the range up to 300 °C with mass losses 13.69% and 2.74%. The next step occurs over a range 300–625 °C with a mass loss of 71.43% followed by metal oxide formation.
[CoH2Lb(H2O)2]ClO4 · 2H2O complexes
For the anhydrous complex formed at 220 °C, there are three decomposition steps shown in Table 4. One step occurs at 300–360 °C with mass loss 14.07%. The next steps of partial decomposition occur at 360–550 °C and 550–790 °C with mass loss 28.37% and 35.65%, respectively, with the formation of metallic residue 11.73% of Co2O3.
Kinetics and thermodynamic parameters
The C s values (mass fraction at T s) of thermal decomposition of the complexes are in the range 0.28–0.37 (Table 5). This indicates that the decomposition follows first order kinetics [44]. The calculated values of E*, A, ΔS*, ΔH* and ΔG* for the decomposition steps are given in Table 5. For the chromium complexes, [CrH2La(OH)(H2O)] · 3H2O complex showed considerable thermal stability as reflected from the high value of activation energy averaged to 81 KJ mol−1. While [CrH2LbCl(H2O)] · 3H2O complex showed moderate thermal stability as reflected from the activation energy value 41.5. For iron complexes [FeH2La(OH)] · 3H2O and [FeH2Lb(OH)(H2O)] · 5H2O showed moderate thermal stability as shown in Table 5, E* ranging from 20.4 to 81.2 KJ mol−1. With respect to cobalt complexes the Co(II) complex [CoH2La(H2O)2] · 4H2O the E* ranging from 42.5 to 73 KJ mol−1 reflects moderate stability while Co(III) complex [CoH2Lb (H2O)2]ClO4 · 2H2O showed high thermal stability which reflected from the energies of activation ranging from 38.8 to 107 KJ mol−1.
In general and based on the sum of the energies of activation, the H4La complexes are thermally more stable than the H4Lb complexes; this may be due to that the aliphatic chain (CH2)2 in H4La is shorter than chain (CH2)3 in H4Lb. It is well known that Co(III) complexes are inert and stable, so the E* of [CoH2Lb(H2O)2]ClO4 · 2H2O complex is higher than that of the other studied complexes.
The entropy change ΔS* for the complex formation from the starting reactants is negative for all the studied complexes. This means that the decomposition reactions are slow [45].
Considering the TG results, some remarks and conclusions can be drawn as follows:
-
1.
There are two kinds of H2O molecules i.e. hydrated and coordinated.
-
2.
The thermal decomposition of complexes includes several steps.
-
3.
A coordination number 6 is proposed for these metal ions considering the H4L(a) and H4L(b) as tetradentate ligands.
Geometrical structure of the complexes
Concerning the ligating properties of the Schiff bases and the geometry of their metal complexes, the following conclusions can be drawn:
-
(a)
The IR spectral data indicate that the Schiff bases H4La and H4Lb act as dinegative N2O2 tetradentate ligands. The bonding occur via two azomethine N atoms and two phenolic O atoms in position 7 (and not in position 5) to form 1:1 M/L complex.
-
(b)
From the electronic spectral data and magnetic moment values, a square planar structure has been proposed for [Zn L]. Whereas, the results of the electronic absorption spectra of the chelates of the type [ML(H2O)2] and [MLX(H2O)] (M = Cr(III), Fe(III), Co(III)) agree with an octahedral environment around the metal ion as in Fig. 2. The dinegative tetradentate Schiff base lies in the equatorial plane while 2 H2O (or H2O + anion Y) occupy the two axial positions. Moreover, the Co(III)–H2Lb complex behaves as a cationic complex and its molar conductance is in agreement with the 1:1 electrolyte. Based on the above discussion, the structure of the octahedral complexes may be represented in Fig. 2
Fig. 2 Octahedral structure of 1:1 chelates [M(H2L)(H2O)Y] · nH2O, where Y = OH or Cl anion as shown in Table 1
References
Lancashire RL, Smith TD, Pilbrow R. An electron spin resonance study of substituent effects in adducts of cobalt (II) Schiff base complexes with dioxygen. J Chem Soc Dalton Trans. 1979;1:66–70.
Nishida Y, Kida S. Splitting of d orbitals in square planar complexes of copper(II), nickel(II) and cobalt (II). Coord Chem Rev. 1980;27:275–348.
Kanda W, Okawa H, Kida S. Synthesis and characterization of the diamagnetic CoIII–O2 complex with a salen analogue containing a pendant phenolic group. J Chem Soc Chem Commun. 1983:973–4.
Nishinaga A, Tojo T, Matsuura T. A model catalytic oxygenation for the reaction quercetinase. J Chem Soc Chem Commun. 1974:896–7.
Nishinaga A, Tomita H, Nishizawa K, Matsuura T. Regioselective formation of peroxyquinolatocobalt(III) complexes in the oxygenation of 2,6-di-t-butylphenols with cobalt(II) Schiff base complexes. J Chem Soc Dalton Trans. 1981:1504–14.
Zeng W, Li J, Mao Z, Hong Z, Qin S. Synthesis, oxygenation and catalytic oxidation performance of crown ether containing Schiff base transition metal complexes. Adv Synth Catal. 2004;346(11):1385–91.
Kuraoka K, Yazawa T, Chujo Y. A novel inorganic-organic hybrid membrane for oxygen/nitrogen separation containing a cobalt (II) Schiff base complex as oxygen carrier using poly(N-vinylpyrrolidone) as mediator. Chem Commun. 2000;24:2477–8.
El-Wahab ZH, Mashaly MM, Salma AA, El-Shetary BA, Faheim AA. Co(II), Ce(III) and UO2(VI) bis-salicylatothiosemicarbazide complexes: binary and ternary complexes, thermal studies and antimicrobial activity. Spectrochim Acta. 2007;60(12):2861–73.
El-Table AS, El-Enein SA. Reactivity of the new potentially binucleating ligand, 2-(acetichydrazido-N-methylidene-∞-naphthol)-benzothiazol, towards manganese (II), nickel (II), cobalt (II), copper (II) and zinc (II) salts. J Coord Chem. 2004;57:281–94.
Nagpal P, Singh RV. Toxicological effects, biological aspects and spectral characterization of organoboron(III) complexes of sulfonamide-imines. Appl Organomet Chem. 2004;18:221–6.
Yildiz M, Duelger B, Koyuncu SY, Yapici BM. Synthesis and antimicrobial activity of bis (imido) Schiff bases derived from thiosemicarbazide with some 2-hydroxyaldehydes and metal complexes. J Indian Chem Soc. 2004;81:7–12.
Majeti NN, Kumar R. A review of chitin and chitosan applications. React Funct Polym. 2000;46(1):1–27.
Vaidyanathan VG, Weyhermullar T, Nair BU, Subramanian J. DNA damage induced by a chromium (III) Schiff base complex is reversible under physiological condition. J Inorg Biochem. 2005;99(11):2248–55.
Vijayalakshmi R, Kanthimathi M, Subramanian V, Nair BU. Interaction of DNA with [Cr (Schiff base)(H2O)2] ClO4. Biochem Biophys Acta (BBA). 2000;1475(2):157–62.
Soliman AA, El-Medani SM, Ali OAM. Thermal study of chromium and molybdenum complexes with some nitrogen and nitrogen-oxygen donors ligands. J Therm Anal Calorim. 2006;83(2):385–92.
Issa RM, Amer SA, Mansour IA, Abdel-Monsef AI. Thermal studies of bis salicylidene adipic dihydrazone derivatives and their complexes with divalent ions of Mn, Co, Ni, Cu and Zn. J Therm Anal Calorim. 2007;90(1):261–7.
Mishra AK, Mishra SB, Manar N, Kaushik NK. Thermal and spectral studies of palladium (II) complexes. J Therm Anal Calorim. 2007;90(2):509–15.
Modi CK, Thaker BT. Some novel tetradentate Schiff base complexes VO(IV) and Cu(II) involving fluorinated heterocyclic β-diketones and polymethylene diamines of varying chain length. J Therm Anal Calorim 2008;94(2):567–77.
Lyszczek R. Thermal and spectroscopic investigation of new lanthanide complexes with 1,2,4-benzenetricarboxylic acid. J Therm Anal Calorim. 2007;90(2):533–9.
Kose DA, Necefoglu H. Synthesis and characterization of bis (nicotinamide) m-hydroxybenzoate complexes Co(II), Ni(II), Cu(II) and Zn(II). J Therm Anal Calorim. 2008;93(2):509–14.
Sherif OE, Issa YM, Abbas SM. Thermodynamic parameters of some Schiff bases derived from 5,7-dihydroxy-6-formyl-2-methylbenzopyran-4-one. J Therm Anal Calorim. 2000;59:913–26.
Nawrot-Modranka J, Nawrot E, Graczyk J. Corrigendum to “in-vivo antitumor, in vitro antibacterial activity and alkylating properties of Phosphorohydrazine derivatives of coumarin and chromone”. Eur J Med Chem. 2006;41:1301–9.
Garhwal S, Sandhu JS. Indium triflate: a new catalyst for (4+2)-cycloaddition of chromone Schiff's bases. J Chem Soc Perkin Trans. 2000;1:2827–9.
Wang B, Yang Z, Qin D, Chen Z. Synthesis, characterization, cytotoxic activity and DNA binding properties of the Ln(III) complexes with ethylene diaminobis(6-hydroxychromone-3-carbaldehyde) Schiff base. J Photochem Photobiol A. 2008;194:49–58.
Emeleus HJ, Sharpe AG. Modern aspects of inorganic chemistry. 4th ed. London: Roultedge and Paul; 1974. p. 480.
Schonberg A, Badran N, Strakousky NA. Furo-chromones and -coumarins. VII. Degradation of Visnagin, Khellin and related substances; experiments with chromic acid and hydrogen peroxide; and a synthesis of Eugenitin. J Am Chem Soc. 1953;75:4992–5.
El-Ansary AL, Abdel-Fattah HM, Abdel-Kader NS. Elucidation of molecular structure of new Schiff bases derived from benzopyran-4-one using spectroscopic techniques. Egypt J Anal Chem. 2006;15:141–9.
Macdonald AMG, Sirichanya P. The determination of metals in organic compounds by oxygen-flask combustion or wet combustion. Microchem J. 1969;14:199–206.
Horowitz HH, Metzger G. A new analysis of thermogravimetric traces. Anal Chem. 1963;35:1464–8.
Coats AW, Redfern JP. Kinetic parameters from thermogravimetric data. Nature. 1964;201:68–9.
Nakamoto K. Infrared spectra of inorganic and coordination Compounds. 2nd ed. New York: Wiley Interscience; 1970.
Emara AAA, Khalil SME, Salib KAR. Di-, tri- and poly-nuclear transition metal complexes of 3,4-diacetyl-2,5-hexanedione. J Coord Chem. 1995;36:289–301.
Abd El-Zaher MM. Spectroscopic characterization of some tetradentate Schiff bases and their complexes with nickel, copper and zinc. J Chin Chem Soc. 2001;48:153–8.
Abu El-Wafa SM, El-Behairy MA, Issa RM. Preparation and characterization of Co(III) complexes of tetradentate N2O2 Schiff base ligands. IR, electronic, 13C NMR, conductance and magnetic studies. Bull Soc Chim Fr. 1987:445.
Liehr AD. The three electron (or hole) cubic ligand field spectrum. J Phys Chem. 1963;67:1314–28.
Chandra S, Pendeya KB Singh RP. Some chromium complexes of cyclo-hexanone semicarbazone and cyclohexanone thiosemicarbazone. J Inorg Nucl Chem. 1980;42:1075–8.
Geogrieva I, Trendafilova N, Bauer G. Spectroscopic and thyeoretical study of Cu(II), Zn(II), Ni(II),Co(II) and Cd(II) complexes of glyoxilic acid oxime. Spectrochim Acta A. 2006;63:403–15.
Jolly WL. Principles of inorganic chemistry. London: Mc Graw-Hill; 1976. p. 272.
Ellis GP, Williams JM. Benzopyrones. Part 18. Carbon-13 nuclear magnetic resonance spectroscopy of chromones. J Chem Soc Perkin Trans. 1981;19:2557–60.
Shallaby AM, Mostafa MM, Bekheit MM. Complexes of Cu(II), Co(II), Ni(II), Cd(II) and Hg(II) halides with hydrazones derived from girard T and chromone. J Inorg Nucl Chem. 1979;41(2):267–9.
Geary WJ. The use of conductivity measurements in organic solvents for the characterization of coordination compounds. Coord Chem Rev. 1971;7:81–122.
Birzescu M, Niculescu M, Dumitru R, Budrugeac P, Segal E. Copper (II) oxalate obtained through the reaction of 1,2-ethanediol with Cu(NO3)2·3H2O. Structural investigations and thermal analysis. J Therm Anal Calorim. 2008;94(1):297–303.
Ispir E, Serin S. New immobilized Schiff bases. Synthesis, complexation, characterization and thermal behaviors. J Therm Anal Calorim. 2008;94(1):281–8.
Nathg M, Arora P. Spectral and thermal studies of cobalt(II), nickel(II) and copper(II) complexes of Schiff bases obtained from o-hydroxyacetophenone and amino acids. Synth React Inorg Met Org Chem. 1993;23:1523–45.
Valaev LT, Gospodinov GG. Study on the kinetics of the isothermal decomposition of selenites from IIIB group of the periodic system. Thermochim Acta. 2001;370:15–9.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abdel-Fattah, H.M., El-Ansary, A.L. & Abdel-Kader, N.S. Thermal and spectral studies on complexes derived from tetradentate Schiff bases. J Therm Anal Calorim 96, 961–969 (2009). https://doi.org/10.1007/s10973-009-0062-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-009-0062-z