Abstract
To develop high performance mixed matrix gel polymer electrolyte, the synergistic effect of blending PVDF (PEO/PVDF weigh ratio of 85:15) and adding nano-TiO2 (0.5–2 wt.%) to traditional monomer poly (ethylene oxide) was investigated. Thermogravimetric analysis indicated that poly (ethylene oxide)/poly (vinylidene fluoride) blend had an excellent thermal performance. X-ray diffraction analysis revealed that poly (ethylene oxide)/poly (vinylidene fluoride) blend leads to lower crystallinity and the amorphicity of corresponding gel polymer electrolyte increases with increasing nano-TiO2 concentration. The maximum ionic conductivity (2.12 × 10−6 and 6.37 × 10−6 S/cm) of poly (ethylene oxide)/-(TiO2)0.5 and poly (ethylene oxide)/poly (vinylidene fluoride)/-(TiO2)0.5 gel polymer electrolyte at room temperature (25 °C) were obtained, respectively. The prepared poly (ethylene oxide)/poly (vinylidene fluoride)-TiO2 gel polymer electrolyte demonstrated about 2.6 and 1.8-fold increment in the fracture strength as compared to that of poly (ethylene oxide) gel polymer electrolyte and poly (ethylene oxide)-TiO2 gel polymer electrolyte. The average transmittance of poly (ethylene oxide)/poly (vinylidene fluoride)-TiO2 gel polymer electrolyte was about 90 % in the visible region. With good electrical, mechanical and optical performance, it is very suitable for being applied in electrochromic glass.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Gel polymer electrolytes (GPEs) are considered as an important class of electrolyte materials for the construction of a new generation of electrochemical devices because they have distinct advantages over liquid electrolytes due to their low reactivity with electrodes, reduced risk of leakage, flexibility and improved integrity. GPEs have been widely explored for their favorable mechanical properties, ease of fabrication and handling in thin films, modularity and reliability in various electrochemical devices and their ability to form effective electrode-electrolyte contact [1–5]. For the GPEs, however, it is difficult to exhibit high ionic conductivity and good mechanical properties simultaneously for the polymer matrix formed by one monomer, i.e., poly (ethylene oxide) (PEO), poly (vinylidene fluoride) (PVDF) [6], polyacrylonitrile [7], poly (methyl methacrylate) (PMMA) [8, 9]. In which, PEO has good solubility for the lithium salt and PEO plays an important role in the development of gel polymer-based electrolytes for its low cost, environmental stability, safety, facile synthesis [10], and high electrochemical stability in comparison with other polyethers or co-polymers. However, low ionic conductivity and poor mechanical property of PEO-based GPEs limit their potential practical applications in devices at ambient temperature.
Various methods have been performed to enhance the ionic conductivity and mechanical properties of PEO-based GPEs [11]. One of the methods used for increasing the ionic conductivity is to suppress the crystallization of PEO chains, leading to an increase in the chain mobility [12]. The crystallization of PEO can be suppressed by using a polymer blend [13], nanocomposites [14], crosslinking [15], and/or copolymerization [16, 17]. Blending of different polymers provides easy preparation and feasible control of the physical properties within the miscibility compositional region [18]. It also exhibits properties that are superior to the properties of individual component of the blend [19–21]. PVDF is widely used as a membrane material since it exhibits excellent chemical resistance, good physical, thermal stability [22], high dielectric constant (ε = 8.4) and strong electron-withdrawing functional groups (–C–F). Fan et al. [23] reported that the blend of PEO and PVDF could hinder the crystallinity of PEO, and achieve a good combination of high ionic conductivity and good mechanical strength. Muthuvinayagam et al. [24] revealed PVDF/PVA-based polymer electrolytes obtained high ionic conductivity and other properties.
Another feasible approach has been used to improve the ambient temperature conductivity while retaining the mechanical properties and the stability of GPE by the addition of nano-scale ceramic filler such as Al2O3 [25], SiO2 [26], TiO2 [27] and BaTiO3 [28]. Liang et al. [29] studied the effect of Al2O3 on the electrical and mechanical performance of PEO/PMMA-based polymer electrolyte membrane, which showed the ionic conductivity and fracture stress of the membrane were improved to 9.37 × 10−7 S/cm and 3.26 MPa, respectively. Ni’mah et al. [30] prepared the polymer electrolytes based on polyethylene oxide (PEO) with NaClO4 salt and nano-sized TiO2 fillers. The result showed the TiO2-blend electrolyte had higher amorphicity and ionic conductivity.
The blend and the addition of fillers improve several properties of GPE, such composite electrolytes have been almost explored for Li-ion batteries and supercapacitor, but rarely in the field of electrochromic glass. Few studies have reported positive effects on parameters such as thermal stability, mechanical properties and optical performance. In the present study, PEO is blended with PVDF polymer at PEO/PVDF weigh ratio of 85:15 with the aim to decrease the crystallinity of PEO matrix and improve the mechanical property. Nano-TiO2 supports the ionic mobility due to its substrate characteristics, such as shape and surface nature, which effectively disturbs the order packing tendency of the polymer chains [31]. Besides that, nano-TiO2 particles could effectively absorb the ultraviolet ray in visible light that is harmful to human body. Nano-TiO2 is further incorporated into PEO/PVDF system in order to enhance the ionic conductivity and mechanical property of corresponding GPE. Materials characterization in terms of thermal stability, degree of crystallinity, morphology, electrical performance, mechanical property and transmittance of PEO/PVDF-(TiO2)x (x = 0–2.0 wt.%) GPE prepared by the solution casting method were systematically investigated in details.
2 Experimental
2.1 Materials
PEO (M W = 1 × 106) and PVDF (America Solvay 6020) which were dried at 60 °C under vacuum for 24 h were purchased from Sigma-Aldrich (St. Louis, MO, USA). Lithium perchlorate (LiClO4, 99.99 %), which was dried in vacuum at 70 °C for 24 h, was fabricated by the Shanghai Aladdin Biological Technology Company (Shanghai, China). Titanium dioxide nanoparticles (commercial P25) were obtained from Degussa Co (Germany). N,N-dimethylacetamide (DMAC, 99.5 %) was acquired from Tianjin Kemiou Chemical Reagent (Tianjin, China). All chemicals were used as received without further purification.
2.2 Preparation of PEO/PVDF-TiO2 GPE films
Preparation of GPE used the solution casting technique. PEO and PVDF, whose weight ratio was kept at 85:15, were dissolved as matrix in DMAC with a different weight ratio TiO2 (x = 0.5–2 wt.%, x is the percentage of PEO and PVDF total quality) in a magnetic stirrer for 15 min. Then, 10 wt.% LiClO4 of PEO/PVDF total weight was added to the above solution. After stirring for 5 h at 50 °C, the obtained homogenous mixture was cast onto a Teflon plate and DMAC was evaporated slowly in the dry oven at 40 °C for 3–4 h, then GPE film was obtained. The obtained film was dried in vacuum for 24 h at 60 °C. The thickness of the film was about 30 μm. The films of the other GPE were prepared in the same way. Fig. 1
2.3 Characterization
Thermal stability of GPE matrixes was investigated using (SDT Q600 TGA/DTA apparatus (TA, USA) at a heating rate of 12 °C min-1 from 20 to 600 °C under argon atmosphere. The sample weights were maintained in the range of 8–9 mg. D8 advance X-ray diffractometer (Bruker axes, USA) was applied to investigate the amorphousness at a scan rate of 5°/min, in the 2θ range from 5° to 40° and the crystallinity of the samples was calculated by Jade software. The morphologies and the thickness of the films were investigated using a field-emission scanning electron microscopy which was studied by S4800 model (Hitachi, Japan). The electrochemical impedance spectra of GPE was measured at room temperature (25 °C) by electrochemical work station (Zahner Zennium) in the frequency range from 0.1 to 100 KHz and the signal amplitude was 10 mv. GPE film was made into a button battery to test the bulk resistance. The ionic conductivity of the films σ was calculated by the equation:
where d and S are the thickness of the films and the area of the films respectively and R b is the bulk resistance. The equivalent circuit was fitted by the ZView software and the resistance of the electrolyte (R b) was determined from the intercept of the real impedance. Transmittance spectra of the samples were recorded on a Lambda 35 UV-Vis spectrophotometer (PerkinElmer) at room temperature in the wavelength range of 220–800 nm. LLY-O6A model, a single fiber electronic tensile strength tester (Laizhou, China), had been used to measure the mechanical strength of GPE films with clip speed of 5 mm/min and gauge length of 10 mm. All the tested samples were done in 20 replicates.
3 Results and discussion
3.1 Thermal properties
The thermal stability of GPE matrixes was characterized by thermogravimetric analyzer (TGA) and the results are presented in Fig. 2, which shows the initial 2–3 % weight loss at 220 °C that is mainly due to the evaporation of moisture absorbed by the samples during the process of sample. TGA curves of PEO and PVDF show that the decomposition temperature is about 375 and 460 °C, respectively. There is still 5 and 32 wt.% material left at 600 °C, respectively. Besides, TGA shows that PEO/PVDF blend has intermediate thermal stability of PEO and PVDF polymer. Only one weightlessness peak indicates that PEO and PVDF have a good compatibility and PVDF can be distributed uniformly in PEO. The decomposition temperature of PEO/PVDF blend is about 390 °C and there is about 19 wt.% of the material left, which illustrates the good thermal stability.
3.2 X-ray diffraction (XRD)
Fig. 3 shows the crystallinity changes in the different electrolyte films characterized by means of X-ray diffraction. The values of crystallinity are summarized in Table 1. XRD pattern of bare PEO shows two high intensity diffraction peaks at 19° and 23°, which are assigned to set of planes (120) and (112) [32], respectively. From Fig. 3a and Table 1, blending PVDF into PEO results in decreasing crystallinity (from 73.22 to 62.03 %). Moreover, it is observed that after introducing nano-TiO2 to PEO/PVDF GPE, the degree of crystallinity of PEO/PVDF GPE is reduced further from 59.90 to 35.69 %. This could be due to the disruption of the semi-crystalline structure of the film by nano-TiO2. Low crystallinity of a polymer film favors the chain movement and increases the ionic conductivity of the corresponding polymer electrolyte subsequently [33].
3.3 Morphology characterization
The compatibility between the polymer matrix and the inorganic dopant has great influence on the properties (mechanical and ion conductivity) of the polymer blend electrolytes [34]. SEM images for different GPE films are displayed in Fig. 4. Fig. 4a shows the typical spherulitic texture of the bare PEO, demonstrating its semi-crystalline nature [32]. It can be seen from Fig. 4b that there is no apparent interface between two polymers, which indicates that PEO and PVDF have a good compatibility. There is uniform and smooth surface in PEO/PVDF/LiClO4 film (Fig. 4c). This is because lithium salt could change the surface appearance of PEO/PVDF blend film. From the cross section image of PEO/PVDF/LiClO4 film, inserted in Fig. 3c, we can see that the thickness of GPE film (top) is about 30 μm. Fig. 4d shows that nano-TiO2 particles are distributed on PEO/PVDF GPE film.
3.4 Conductivity studies
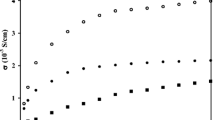
Fig. 5 illustrates the impedance spectra for PEO and PEO/PVDF GPE with different nano-TiO2 contents at room temperature (25 °C). The inset in Fig. 5 is the equivalent circuit of GPE films. Fig. 5b gives the smaller values of bulk resistance which is obtained from the intercept on the real axis of the Nyquist plot of PEO/PVDF-TiO2 GPE compared to PEO-TiO2 GPE. This is mainly the result of lower crystallinity proved in Fig. 3a that contributes to fast Li-ion motion in the polymer network, hence improves the ion conductivity. Fig. 6 shows the ionic conductivity of PEO and PEO/PVDF GPE films with different nano-TiO2 contents. From the graph, when PVDF is blended into PEO-TiO2, the ionic conductivity obviously increases. In particular, the ionic conductivity of PEO/PVDF-TiO2 GPE films gains the highest ionic conductivity (6.37 × 10−6 S/cm) when the amount of nano-TiO2 content increases to 0.5 wt.%. An excess of nano-TiO2 particles could reduce the ionic conductivity, which is considered due to an increase by in ion-ion interaction that blocks the motion of Li+ ions and reduces the conductivity pathway [35]. In addition, we also compare our experimental data of the highest ionic conductivity values of PEO/PVDF-TiO2 GPE films with other reported GPE film as shown in Table 2 [29, 32, 36, 37]. It should be noted that the values and comparisons reported for the ionic conductivity have only a relative meaning because of different testing conditions, type of materials and methods.
3.5 Mechanical strength
To quantify the structural stability of GPE films, the mechanical properties measurements based on tensile stress-strain characteristics are carried out in Fig. 7. As can be seen from Fig. 7, the stress of PEO-TiO2 and PEO/PVDF-TiO2 GPE films firstly increase and then decrease with the increasing of nano-TiO2 particles. Compared with PEO-TiO2 GPE films, PEO/PVDF-TiO2 GPE films clearly display better mechanical strength (see Fig. 8), which means blending PVDF and adding TiO2 to PEO have a synergistic effect on the performance of bare PEO. The prepared PEO/PVDF-TiO2 demonstrates about 2.6 and 1.8-fold increment in the fracture strength as compared to that of PEO GPE and PEO-TiO2 GPE, which may be contributed to the C–F bond and hydrogen ion that form the most stable and sturdy construction.
3.6 Optical properties
Due to the kind of electrolyte film for electrochromic glass, the transmittance exhibited is quite vital. In visible region, the average transmittances of PEO/PVDF with 0 wt., 0.5 wt., 1.0 wt., 1.5 wt. and 2.0 wt.% TiO2 are about 96.19, 93.66, 92.70, 90.58 and 89.84 %, respectively, (see Fig. 9), suggesting that increasing nano-TiO2 content has a negative effect on the transmittance. However, it is clear that PEO/PVDF-(TiO2)(0.5–2.0) GPE films in the visible range still have high transmittance which proves the practical applications of GPE films on the electrochromic glass.
4 Conclusions
Gel polymer electrolyte (GPE) based on PEO/PVDF blend with different weight percent of nano-TiO2 fillers had been prepared by solution casting method. PEO/PVDF was stable up to 390 °C before decomposition. PVDF and nano-TiO2 could decrease the crystallinity of bare PEO and PEO/PVDF GPE films, respectively. Moreover, the blend of PVDF into PEO-TiO2 GPE films enhanced the ionic conductivity and mechanical strength. The maximum value of ionic conductivity of PEO/PVDF-TiO2 GPE was 6.37 × 10−6 S/cm at room temperature (25 °C) when the amount of nano-TiO2 content was 0.5 wt.%. The blending PVDF and adding TiO2 had a synergistic effect on the performance of bare PEO. The prepared PEO/PVDF-TiO2 GPE demonstrated about 2.6 and 1.8-fold increment in the fracture strength as compared to that of PEO GPE and PEO-TiO2 GPE. The average transmittance of PEO/PVDF-TiO2 GPE film in visible region was about 90 %, which met the actual demand of electrochromic glass.
References
Rao CVS, Ravi M, Raja V, Bhargav PB, Sharma AK, Rao VN (2012) Preparation and characterization of PVP-based polymer electrolytes for solid-state battery applications. Iran Polym J 21:531–536
Yap YL, You AH, Teo LL, Hanapei H (2013) Inorganic filler sizes effect on ionic conductivity in polyethylene oxide (PEO) composite polymer electrolyte. Int J Electrochem Sci 8:2154–2163
Stephan AM, Kumar SG, Renganathan NG, Kulandainathan MA (2005) Characterization of poly (vinylidene fluoride–hexafluoropropylene) (PVDF-HFP) electrolytes complexed with different lithium salts. Eur Polym J 41:15–21
Hashmi SA (2004) Supercapacitor: an emerging power source. Natl Acad Sci Lett 27:27–46
Goodenough JB, Kim Y (2009) Challenges for rechargeable Li batteries. Chem Mater 22:587–603
Li ZH, Zhang P, Zhang HP, Wu YP, Zhou XD (2008) A lotus root-like porous nanocomposites polymer electrolyte. Electrochem Commun 10:791–794
Yang CR, Perng JT, Wang YY, Wan CC (1996) Conductive behaviour of lithium ions in polyacrylonitrile. J Power Sources 62:89–93
Gao K, Hu X, Yi TF, Dai C (2006) PE-g-MMA polymer electrolyte membrane for lithium polymer battery. Electrochim Acta 52:443–449
Appetecchi GB, Croce F, Scrosati B (1995) Kinetics and stability of the lithium electrode in poly (methyl methacrylate)-based gel electrolytes. Electrochim Acta 40:991–997
Nadimicherla R, Kalla R, Muchakayala R, Guo X (2015) Effects of potassium iodide (KI) on crystallinity, thermal stability, and electrical properties of polymer blend electrolytes (PVC/PEO:KI). Solid State Ionics 278:260–267
Polu AR, Rhee HW (2015) Nanocomposite solid polymer electrolytes based on poly(ethylene oxide)/POSS-PEG (n=13.3) hybrid nanoparticles for lithium ion batteries. J Ind Eng Chem 31:323–329
Park CH, Lim JY, Lee JH, Lee JM, Park JT, Kim JH (2016) Synthesis and application of PEGBEM-g-POEM graft copolymer electrolytes for dye-sensitized solar cells. Solid State Ionics 290:24–30.
Deng F, Wang X, He D, Hu J, Gong C, Ye YS, Xue Z (2015) Microporous polymer electrolyte based on PVDF/PEO star polymer blends for lithium ion batteries. J Membrane Sci 491:82–89
Cheng S, Smith DM, Li CY (2015) Anisotropic ion transport in a Poly (ethylene oxide)-LiClO4 solid state electrolyte templated by graphene oxide. Macromolecules 48(13):4503–4510
Zhao H, Ding X, Yang P, Li L, Li X, Zhang Y (2015) A novel multi-armed and star-like poly (ethylene oxide) membrane for CO2 separation.J Membr Sci 489:258–263
Jeon H, Lee CS, Patel R, Kim JH (2015) Well-organized meso-macroporous TiO2/SiO2 film derived from amphiphilic bubbery comb copolymer. ACS Appl Mater Inter 7(14):7767–7775
Chi WS, Kim SJ, Lee SJ, Bae YS, Kim JH (2015) Enhanced performance of mixed-matrix membranes through a graft copolymer-directed interface and interaction tuning approach. ChemSusChem 8(4):650–658
Lee L, Park SJ, Kim S (2013) Effect of nano-sized barium titanate addition on PEO/PVDF blend-based composite polymer electrolytes. Solid State Ionics 234(10):19–24
Tang M, Liau WR (2000) Solvent effect on the miscibility of poly (4-hydroxystyrene)-poly (ethylene oxide) blends. Eur Polym J 36(12):2597–2603
Bai BC, Kim JG, Im Ji S, Lee YS (2011) The hydrogen storage capacity of metal-containing polyacrylonitrile-based electrospun carbon nanofibers. Carbon Lett 12(3):171–176
Rocco AM, Pereira RP, Felisberti MI (2001) Miscibility, crystallinity and morphological behavior of binary blends of poly (ethylene oxide) and poly (methyl vinyl ether-maleic acid). Polymer (Guildf) 42(12):5199–5205
Tamilselvi P, Hema M (2014) Conductivity studies of LiCF3SO3 doped PVA:PVdF blend polymer electrolyte. Physica B: Condensed Matter 437:53–57
Fan L, Dang Z, Nan CW, Li M (2002) Thermal, electrical and mechanical properties of plasticized polymer electrolytes based on PEO/P(VDF-HFP) blends. Electrochim Acta 48:205–209
Muthuvinayagam M, Gopinathan C (2015) Characterization of proton conducting polymer blend electrolytes based on PVDF-PVA. Polymer (Guildf) 68:122–130
Liao Y, Chen T, Luo X, Fu Z, Li X, Li W (2016) Cycling performance improvement of polypropylene supported poly (vinylidenefluoride-co-hexafluoropropylene)/maleic anhydride-grated-polyvinylidene fluoride based gel electrolyte by incorporating nano-Al2O3 for full batteries. J Membr Sci 507:126–134
Liu S, Imanishi N, Zhang T, Hirano A, Takeda Y, Yamamoto O, Yang J (2010) Effect of nano-silicafiller in polymer electrolyte on Li dendrite formation in Li/poly (ethylene oxide)–Li(CF3SO2)2 N/Li. J Power Sources 195(19):6847–6853
Chung SH, Wang Y, Persi L, Croce F, Greenbaum SG, Scrosati B, Plichta E (2001) Enhancement of ion transport in polymer electrolytes by addition of nanoscale inorganic oxides. J Power Sources 97:644–648
Vickraman P, Senthilkumar V (2010) A study on the role of BaTiO3 in lithum bis (perfluoroethanesulfonyl) imide-based PVDF-HFP nanocomposites. Ionics 16(8):763–768
Liang B, Tang S, Jiang Q, Chen C, Chen X, Li S, Yan X (2015) Preparation and characterization of PEO-PMMA polymer composite electrolytes doped with nano-Al2O3. Electrochim Acta 169:334–341
Ni’mah YL, Cheng MY, Cheng JH, Rick J, Hwang BJ (2015) Solid-state polymer nanocomposite electrolyte of TiO2/PEO/NaClO4 for sodium ion batteries. J Power Sources 278:375–381
Kim KS, Park SJ (2012) Influence of N-doped TiO2 on lithium ion conductivity of porous polymeric electrolyte membrane containing LiClO4. Solid State Ionics 212:18–25
Polu AR, Rhee HW (2016) Effect of TiO2 nanoparticles on structural, thermal, mechanical and ionic conductivity studies of PEO12-LiTDI solid polymer electrolyte. J Ind Eng Chem 37:347–353
Xie H, Liao Y, Sun P, Chen T, Rao M, Li W (2014) Investigation on polyethylene-supported and nano-SiO2 doped poly (methyl methacrylate-co-butyl acrylate) based gel polymer electrolyte for high voltage lithium ion battery. Electrochim Acta 127:327–333
Reddeppa N, Sharma AK, Rao VVRN, Chen W (2014) AC conduction mechanism and battery discharge characteristics of (PVC/PEO) polyblend films complexed with potassium chloride. Measurement 47:33–41
Klongkan S, Pumchusak J (2015) Effects of nano alumina and plasticizers on morphology, ionic conductivity, thermal and mechanical properties of PEO-LiCF3SO3 solid polymer electrolyte. Electrochim Acta 161:171–176
Klongkan S, Pumchusak J (2015) Effects of the addition of LiCF3SO3 salt on the conductivity, thermal and mechanical properties of PEO-LiCF3SO3 solid polymer electrolyte. Int J Chem Eng Appl 6(3):165
Moreno M, Quijada R, Santa Ana MA, Benavente E, Gomez-Romero P, González G (2011) Electrical and mechanical properties of poly (ethylene oxide)/intercalated clay polymer electrolyte. Electrochim Acta 58:112–118
Acknowledgments
The authors gratefully acknowledge the financial support by the Program for the Science and Technology Plans of Tianjin (No. 16JCTPJC44300 and 15PTSYJC00240), Changjiang Scholars and Innovative Research Team in University (PCSIRT) of Ministry of Education of China (Grand no. IRT13084).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Chen, P., Liang, X., Wang, J. et al. PEO/PVDF-based gel polymer electrolyte by incorporating nano-TiO2 for electrochromic glass. J Sol-Gel Sci Technol 81, 850–858 (2017). https://doi.org/10.1007/s10971-016-4235-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-016-4235-5