Abstract
Pure orthorhombic phase of La1−xSmxFeO3 (x = 0, 0.1, 0.2, and 0.3) nanoparticles can be obtained by sol–gel method after calcination at 800 °C for 3 h in air. X-ray diffraction, scanning electron microscopy, transmission electron microscopy, X-ray photoelectron spectroscopy, Fourier transform infrared spectroscopy, X-ray absorption near edge spectroscopy, ultraviolet-visible spectroscopy, and vibrating sample magnetometry were used to study the crystal structure, morphology, oxidation state, functional group, optical, and magnetic properties of samples. Pure orthorhombic phase of perovskite structure is confirmed by X-ray diffraction results. Decreasing lattice parameters, crystallite sizes, and cell volumes with increasing microstrains indicate structure distortion due to the substitution of Sm ions with small ionic radius on the La sites in the orthorhombic structure. Scanning electron microscopy and transmission electron microscopy images show a homogeneous distribution of almost spherical nanoparticles with decreasing average particle sizes ranging from 56.48 ± 3.22 to 23.21 ± 4.40 nm for samples of high Sm content. Fourier transform infrared spectroscopy spectra confirm the Fe–O stretching mode in octahedral FeO6 unit of a perovskite structure. X-ray photoelectron spectroscopy and X-ray absorption near edge spectroscopy results indicate the oxidation states +3 of La and Fe ions. The optical band gaps are found to decrease from 2.218 to 1.880 eV with increasing Sm content. vibrating sample magnetometry results show the antiferromagnetic behavior of undoped sample and ferromagnetic behavior for doped samples, affecting by structure distortion and particle size reduction. Interestingly, the coercive field is significantly enhanced from 95.07 Oe (x = 0.1) to 13,062.79 Oe (x = 0.3). Curie temperature (T c) is suggested to be above 400 K.
Graphical Abstract
The magnetization curves of La1-xSmxFeO3 (x = 0.0, 0.1, 0.2, and 0.3) nanoparticles prepared by the sol-gel method with the inset show the comparing coercive forces (Hc) of the present work and the previous works, La0.7M0.3FeO3 (M = Al and Ga). Sm-doped LaFeO3 nanoparticles can exhibit ferromagnetic behavior with the significant enhancement of Hc from 95.07 to 13,062.79 Oe. 
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Pure and doped lanthanide orthorhombic perovskites, such as LaFeO3, BiFeO3, GdFeO3, NdFeO3, SmFeO3, and etc. [1–3] continue to attract significant attention as a promising material for applications in solid oxide fuel cells [4], catalysts [5], chemical sensors [6], thermoelectric [7], and magnetic materials [8–10]. Among these materials, lanthanum orthoferrite (LaFeO3) is the common one that has been widely studied because it can exhibit ferroelectric and ferromagnetic properties [11] similar to those observe in BiFeO3 [12, 13]. However, bulk LaFeO3 is known to be an antiferromagnetic material with a very high Néel temperature (T N) of 738 K [14] owing to the stability of a magnetic structure of the collinear arrangement of FeO6 octahedral units in the two interpenetrating pseudo cubic face-centered sublattices [15–19]. Therefore, many researchers attempt to improve its magnetic property by replacement of La and/or Fe sites with various metal ions. For instance, ferromagnetic behavior can be obtained in La1−xAlxFeO3 [20], La1−xGaxFeO3 [21], LaFe1-xMgxO3 [2], La1−xCaxFeO3 [22], La1−xCexFeO3 [23], La1−xCuxFeO3 [24], La1−xPbxFeO3 [25], and LaFe1-xTixO3 [26]. These substitutions induced the distortion of the structure and the reduction of particle size, resulting in uncompensated surface spins [25, 27]. Normally, pure and doped LaFeO3 can be prepared by various techniques, such as electrospinning [28], hydrothermal [29], microwave-assisted method [30], sonochemical method [31], modified solution combustion method [32], sol–gel [33–35] and polymerization complex method [20, 21]. From these synthesis methods, sol–gel method is widely employed for the synthesis of nanoparticles because of the simplicity, low cost, homogeneous dispersion of nanoparticles and most of all, products of pure phase can be obtained. Sm of smaller ionic radius (0.958 Å) than that of La (1.27 Å), but with the same oxidation state +3 is another interesting lanthanide metal that expect to replace the La site to effect the structure distorsion and improve the magnetic property of LaFeO3 similar to those observe in our previous works [20, 21].
In this work, La1−xSmxFeO3 (x = 0.0, 0.1, 0.2, and 0.3) nanoparticles were prepared by sol–gel method. The structural, morphological, optical, and magnetic properties of the obtained products were studied by X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), Fourier transform infrared spectrophotometry (FTIR), Ultraviolet-visible spectrophotometry (UV–vis), X-ray photoelectron spectroscopy (XPS), X-ray absorption near edge spectroscopy (XANES) and vibrating sample magnetometry (VSM). Interestingly, very high coercive force (H c) can be obtained, comparing with those of our previous works [20, 21].
2 Experimental
In the synthesis of La1−xSmxFeO3 (x = 0.0, 0.1, 0.2, and 0.3) nanoparticles by sol–gel method, the stoichiometric amounts of Fe(NO3)3.9H2O (Kento chemical Co., 99.9 %), LaN3O9.6H2O, (Fluka, 99.0 %), and Sm(NO3)3·6H2O (Aldrich, 99.9 %) by a ratio of 1:1 (Fe:La,Sm) with 40 g of C6H8O7.H2O (VWR International Ltd., 99.7 %), were firstly dissolved in a mixture of 60 ml deionized water and 120 ml of ethylene glycol under a mechanical stirring at room temperature using a magnetic bar. Secondly, this solution was heated at 80 °C under a constant stirring until a gel was formed and dried. The final products were pre-calcined at 400 °C for 3 h in air and ground to fine powders. These powders were further calcined at 800 °C for 3 h in air to obtain the perovskite phase of LaFeO3.
Phase and structure of the products were investigated by XRD (SHIMADZU, XRD-6100) with CuKα 1 radiation (λ = 1.5405 Å). The XRD peaks were indexed according to the standard data of LaFeO3 (JCPDS no. 37–1493). Scanning electron microscope (SEM, 1450VP, LEO UK) and TEM (TECNAI G2 20, FEI) were employed to observe the morphology, particle size determination, and the dispersion of particles. The FTIR spectra of all samples were obtained by FTIR (spectrum one FTIR, Perkin Elmer Instrument, USA) in the wavenumber range from 400 to 4000 cm−1, using the KBr pellet technique. UV–vis (Shimadzu UV-3101PC) was employed for the optical properties study of the products in the wavelength from 200 to 800 nm. X-ray photoelectron spectroscopy (XPS, AXIS Ultra DLD, Kratos Analytical Ltd. Manchester UK) was used to investigate the oxidation state of La, Fe and O ions in the structure of La0.7Sm0.3FeO3 nanoparticles. Fe K-edge XANES spectra of standard metals and samples of x = 0.0 and 0.3 were obtained in a transmission mode at the BL5.2 of Synchrotron Light Research Institute in Nakhon Ratchasima, Thailand. Room temperature magnetizations of all samples were measured by the vibrating sample magnetometer (VSM, Versa LabTM free, Quantum Design USA) in the magnetic field range of ±30 kOe. Temperature variation of the magnetization for sample of x = 0.3 was performed in the field cooling (FC) and zero field cooling (ZFC) modes from 50–390 K under an external magnetic field (H) of 10 kOe.
3 Results and discussion
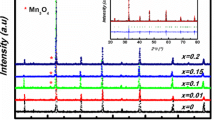
XRD patterns of La1−xSmxFeO3 (x = 0.0, 0.1, 0.2, and 0.3) nanoparticles are shown in Fig. 1. It is obvious that all samples have the perovskite structure of orthorhombic phase as indexed in the standard data of LaFeO3 (JCPDS no. 37–1493). The average crystallite sizes of samples were calculated from the dominant peaks of X-ray line broadening of the (101), (121), (220), (202), and (123) planes using Scherrer equation,
where D Sch is the average crystallite size, θ is the Bragg angle, λ is the wavelength of the X-ray, β is the full width at half maximum, the constant k is taken as 0.9 [36]. The obtained results as summarized in Table 1 show that the average crystallite sizes decrease with increasing Sm content as shown in the inset of Fig. 1 and found to be 58.45 ± 5.90, 30.22 ± 6.32, 23.25 ± 3.64, and 16.96 ± 4.23 nm for samples of x = 0.0, 0.1, 0.2, and 0.3, respectively. The lattice parameters (a, b, and c) and cell volumes of samples were calculated by Rietveld refinement method with the GOF, R wp, and R p in the range of 1.97–2.87 %, 6.09–8.19 %, and 4.53–6.29 %, respectively. All of these parameters are summarized in Table 1. It can be seen that the calculated lattice parameters are in good agreement with those of the orthorhombic phase of LaFeO3 and found to decrease in samples of high Sm content due to the replacement of a larger ionic radius La3+ ion by a smaller ionic radius Sm3+ ion. Similarly, the cell volumes are decreased in the same manner as well. In addition, the crystallite size and the microstrain of samples were also calculated for the comparison by using the Williamson-Hall equation [37],
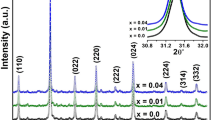
where D WH is the average crystallite size, θ is the Bragg angle, λ is the X-ray wavelength, k = 0.9, β is the full width at half maximum of XRD peaks and ε is the microstrain of the lattice. The plots of βcosθ as a function of sinθ are shown in Fig. 2. D WH and ε of samples are determined from the linear extrapolation and slope of these plots, respectively. All of these values are summarized in Table 1 and it is found that D WH decrease from 60.31 to 28.95 with increasing Sm content, while those of ε increase from 0.0005 to 0.0033 due to the shrinkage of the lattice parameters. These results are in good agreement with the decrease of cell volumes of the La1−xSmxFeO3 (x = 0.0, 0.1, 0.2, and 0.3) crystals.
TEM images with selected area electron diffraction (SAED) patterns of La1−xSmxFeO3 (x = 0.0, 0.1, 0.2 and 0.3) nanoparticles are shown in Fig. 3. It can be seen that all samples compose of almost spherical nanoparticles with the estimated average sizes (D TEM) using Image J program decrease from 56.48 ± 3.22 to 23.21 ± 4.40 nm due to the increase of Sm ion in the structure, as summarized in Table 2. SAED patterns of these samples (insets of Fig. 3) show ring patterns, indicating a polycrystalline of doped samples and they can be indexed to a certain crystalline plane consistent with those of the XRD results. The EDX results in Fig. 3 clearly show that all doped samples contain La, Fe, Sm, and O atoms with high Sm peak intensity in samples of high Sm content. The weight (%) of La, Fe, O, and C atoms, as summarized in Table 2, are quantitatively calculated from the EDX spectra and it is found that La atom decreases in samples of high Sm content, resulting from the substitution of Sm atom on the La site. According to the limitation of our instrument, the weight (%) of Sm can be qualitatively determined, which is found to increase in a proper amount correspond with the doping level of Sm in La1−xSmxFeO3 (x = 0.0, 0.1, 0.2, and 0.3) samples. It can be seen in Table 2 that the weight (%) of Fe atom is insignificantly vary, while those of O and C atoms increase in samples with high amount of Sm atom. The appeared Cu peaks come from the copper grid. Homogeneous distribution of La1−xSmxFeO3 (x = 0.0, 0.1, 0.2, and 0.3) nanoparticles can be observed by SEM images in Fig. 4. The calculated average particle sizes (D SEM) of samples using Image J program are summarized in Table 2. Similarly, D SEM are found to decrease from 57.87 ± 4.88 to 19.84 ± 6.27 nm, which are comparable to D TEM.
FTIR spectra of La1−xSmxFeO3 (x = 0.0, 0.1, 0.2, and 0.3) nanoparticles are shown in Fig. 5. All peaks at ~1492.85 cm−1 are assigned to C=O stretching mode, corresponding to the vibration of a carbonization group. In addition, the absorption peak at ~1635.61 cm−1 is attributed to C–O–C stretching mode, corresponding to the surface-adsorbed oxygen species and organic substances, whereas that at ~3449.94 cm−1 is assigned for the stretching and bending modes of the O–H bond [34, 38]. From these results, it is suggested that heat treatment can promote the carbonization of organic substances as well as the crystallization of nanoparticles. Moreover, the strong absorption peaks at ~563.42 cm−1 and a lower one are attributed to Fe–O stretching vibration, being characteristics of the octahedral FeO6 groups in the perovskite compounds.
The XPS spectra of La0.7Sm0.3FeO3 sample measured for a chemical analysis of surface and the oxidation state of various ions are shown in Fig. 6. The binding energies of La 3d5/2 at 833.726 and 837.943 eV, and La 3d3/2 at 850.431 and 854.843 eV corresponds to La3+ ion, whereas those of Fe 2p3/2 at 710.027 eV and Fe 2p1/2 at 724.051 eV corresponds to Fe3+ ion [21, 35, 38, 39]. The O (1s) main peak at 529.212 eV corresponds to oxygen ion in the perovskite. The O (1s) peaks at 530.304 and 531.471 eV are probably due to an absorbed oxygen (O2−) of water molecule or hydroxyl group (OH−), since the O (1s) binding energy of O2− or OH− ion is generally 2.1–2.5 eV higher than that of lattice oxygen [21, 35, 38]. However, these samples are easily hygroscopic when they are exposed to air, thus the peak at 531.471 eV is considered probably due to a water molecule associated with the surface.
The normalized Fe K-edge XANES spectra of La1−xSmxFeO3 (x = 0.0 and 0.3) nanoparticles and those of Fe-standard metals with different oxidation states are shown in Fig. 7. All XANES spectra at Fe K-edge were measured in a transmission mode at room temperature. It is clearly seen in Fig. 7 that the edge positions of FeO (Fe2+), Fe3O4 (Fe2+, Fe3+), and Fe2O3 (Fe3+) standards appear at approximately 7120, 7122, and 7124 eV, respectively. Clearly, the shift of the edge positions appear at approximately 7126 and 7127 eV for samples of x = 0.0 and 0.3, which close to that of the Fe3+ standard, indicating the valence state of +3 for Fe ion in these samples [2, 21, 26].
The UV–vis spectra of La1−xSmxFeO3 (x = 0.0, 0.1, 0.2, and 0.3) nanoparticles are shown in Fig. 8 with an obviously broad-absorption peak centered at approximately 285 nm. From the plot of (αhν)2 vs. hν, shown by an inset in Fig. 8, the optical band gaps (Eg) of these samples can be determined by extrapolating the slope to the zero value of (αhν)2 and the Eg values are found to be 2.218, 2.198, 1.959, and 1.880 eV for samples of x = 0.0, 0.1, 0.2, and 0.3, respectively. These values are monotonically decreased with the increase of Sm content as summarized in Table 3. It is obvious that Sm doping can significantly decrease the particle sizes and band gaps of LaFeO3. This confirms that Sm3+ ion can affect the optical transition in La1−xSmxFeO3 (x = 0.0, 0.1, 0.2 and 0.3) nanoparticles.
The room temperature magnetizations of La1−xSmxFeO3 (x = 0.0, 0.1, 0.2, and 0.3) samples measured by VSM are shown in Fig. 9. The M-H curve of undoped sample is very narrow, indicating the antiferromagnetic behavior of sample, whereas those of the Sm-doped LaFeO3 samples with x = 0.1, 0.2, and 0.3 show larger loops of magnetizations belong to those of ferromagnetic behavior with increasing magnetizations (M), H c, and remanent magnetizations (M r) as summarized in Table 3. However, the coercive field are significantly increased to 13,062.79 Oe for La0.7Sm0.3FeO3 sample, which is approximately 137 times higher than the value of 95.07 Oe for a sample of x = 0.05. Moreover, this value of 13,062.79 Oe is approximately 2.46 and 2.78 times higher than those obtained values of 5308.4 and 4702.326 Oe in our previous works of La0.7Al0.3FeO3 [20] and La0.7Ga0.3FeO3 [21], respectively. The enhancements of M, H c, and M r are suggested to originate from the structure distortion and size reduction due to the decrease of lattice parameters as a result of Sm doping. These results confirm the important of size effect on ferromagnetic behavior in perovskite oxides [20–26, 40, 41]. The temperature dependence of magnetization behavior of La0.7Sm0.3FeO3 sample under ZFC and FC modes measured in an external magnetic field of 10 kOe from 50 to 380 K are shown in Fig. 10. In Fig. 10, the magnetizations of this sample in both FC and ZFC measurements gradually decrease with the increase of temperature due to the thermal fluctuations causing the randomization of polarization direction of magnetic moment [8, 20]. It can be seen in Fig. 10 that the zero value of both magnetizations cannot be observed in the temperature range of measurement, implying that the Curie temperature (T c) is above 400 K [2, 20, 26].
4 Conclusions
La1-xSmxFeO3 (x = 0.0, 0.1, 0.2, and 0.3) nanoparticles can be successfully prepared by sol–gel method. XRD results indicate the orthorhombic phase of nanoparticles with the decrease of lattice parameters, crystallite sizes, and cell volumes due to the substitution of small ionic radius of Sm3+ ion on the La3+ site in the orthorhombic perovskite structure, resulting in the distortion of the crystal structure and particle sizes reduction. The calculated crystallite sizes of samples by Scherrer equation are consistent with those obtained by Williamson-Hall equation. Williamson-Hall plots show the increase of microstrain in samples of high Sm content as a result of the decrease in lattice parameters. SEM and TEM images show nanoparticles of almost spherical shape in high Sm-doped samples with the decrease of average particle sizes ranging from 57.87 ± 4.88 to 19.84 ± 6.27 nm. The determined optical band gaps decrease from 2.218 to 1.880 eV with increasing Sm content. XPS and XANES results indicate the oxidation state 3+ for La and Fe ions in the perovskite structure. Undoped sample exhibits antiferromagnetic behavior, whereas doped samples exhibit ferromagnetic behavior as confirmed by VSM results. The coercive field can be enhanced to a very high value of 13,062.79 Oe for a sample of x = 0.3 with the estimated Curie temperature (T c) above 400 K.
References
Dixon CAL, Kavanagh CM, Knight KS, Kockelmann W, Morrison FD, Lightfoot P (2015) Thermal evolution of the crystal structure of the orthorhombic perovskite LaFeO3. J Solid State Chem 230:337
Ciambelli P, Cimino S, De Rossi S, Lis L, Minelli G, Porta P, Russo G (2001) AFeO3 (A = La, Nd, Sm) and LaFe1−xMgxO3 perovskites as methane combustion and CO oxidation catalysts: structural, redox and catalytic properties. Appl Catal B 29:239
Phokha S, Pinitsoontorn S, Rujirawat S, Maensiri S (2015) Polymer pyrolysis synthesis and magnetic properties of LaFeO3 nanoparticles. Physica B 476:55
Hao Hung M, Madhava Rao MV, Shyang Tsai D (2007) Microstructures and electrical properties of calcium substituted LaFeO3 as SOFC cathode. Mater Chem Phys 101:297
Wang Y, Yang X, Lu L, Wang X (2006) Experimental study on preparation of LaMO3 (M = Fe, Co, Ni) nanocrystals and their catalytic activity. Thermochim Acta 443:225
Khetre SM, Chopade AU, Khilare CJ, Kulal SR, Jadhav HV, Jagadale PN, Bangale SV, Bamane SR (2014) Ethanol gas sensing properties of nano-porous LaFeO3 thick films. J of Shivaji Uni (Sci & Tech) 41(2):250–5347
Minh DL, Mai Hoa VN, Ngoc Dinh N, Thi Thuy N (2013) Electric and thermoelectric properties of LaFeO3 compounds doped by Ti, Co and Cu ions. VNU J Mathematics Physics 29(3):42
Paul Blessington Selvadurai A, Pazhanivelu V, Jagadeeshwaran C, Murugaraj R, Panneer Muthuselvam I, Chou FC (2015) Influence of Cr substitution on structural, magnetic and electrical conductivity spectra of LaFeO3. J Alloys Compd 646:924
Phokha S, Hunpratup S, Pinitsoontorn S, Putasaeng B, Rujirawat S, Maensiri S (2015) Structure, magnetic, and dielectric properties of Ti-doped LaFeO3 ceramics synthesized by polymer pyrolysis method. Mater Res B 67:118
Thirumalairajan S, Girija K, Mastelar VR, Ponpandian N (2015) Investigation on magnetic and electric properties of morphologically different perovskite LaFeO3 nanostructures. J Mater Sci: Mater Electron. 26:8652–8662
Acharya S, Mondal J, Ghosh S, Roy SK, Chakrabarti PK (2010) Multiferroic behavior of lanthanum orthoferrite (LaFeO3). Mater Lett 64:415
Xu X, Guoqiangn T, Huijun R, Ao X (2013) Structural, electric and multiferroic properties of Sm-doped BiFeO3 thin films prepared by the sol–gel process. Ceram Inter 39:6223
Mazumder R, Ghosh S, Mondal P, Bhattacharya D, Dasgupta S, Das N, Sen A, Tyagi AK, Sivakumar M, Takami T, Ikuta H (2006) Particle size dependence of magnetization and phase transition near TN in multiferroic BiFeO3. J Appl Phys 100:1
Todd MR, Gary LC, James MA (1993) Combined magnetic-dipole and electric-quadrupole hyperfine interactions in rare-earth orthoferrite ceramics. Phys Rev B 48:224
Treves D (1965) Studies on Orthoferrites at the Weizmann Institute of Science. J Appl Phys 36:1033
Kodama RH, Berkowitz AE (1999) Atomic-scale magnetic modeling of oxide nanoparticles. Phys Rev B 59:6321
Lee YC, Parkhomov AB, Krishnan KM (2010) Size-driven magnetic transitions in monodisperse MnO nanocrystals. J Appl Phys 107: 09E124-1-3.
Fujii T, Matsusue I, Nakatsuka D, Nakanishi M, Takada J (2011) Synthesis and anomalous magnetic properties of LaFeO3 nanoparticles by hot soap method. Mater Chem Phys 129:805
Köferstein R, Jäger L, Ebbinghaus SG (2013) Magnetic and optical investigations on LaFeO3 powders with different particle sizes and corresponding ceramics. Solid State Ionics 249:1
Janbutrach Y, Hunpratub S, Swatsitang E (2014) Ferromagnetism and optical properties of La1−xAlxFeO3 nanopowders. Nanoscale Res Lett 9:498
Hunpratub S, Karaphun A, Phokha S, Swatsitang E (2016) Optical and magnetic properties of La1−xGaxFeO3 nanoparticles synthesized by polymerization complex method. Appl Surf Sci 380:52
Barbero B, Gamboa JA, Cadus LE (2006) Synthesis and characterisation of La1-xCaxFeO3 perovskite-type oxide catalysts for total oxidation of volatile organic compounds. Appl Catal B 65:21
Shikha P, Kang TS, Randhawa BS (2015) Effect of different synthetic routes on the structural, morphological and magnetic properties of Ce doped LaFeO3 nanoparticles. J Alloys Compd 625:336
Prasad BV, Rao BV, Narsaiah K, Rao GN, Chen JW, Babu DS (2015) Preparation and characterization of perovskite Cu doped LaFeO3 semiconductor ceramics. IOP Conf Series: Mater Sci Eng 73:012129
Chandrasekhar KD, Mallesh S, Murthy JK, Das AK, Venimadhav A (2014) Role of defects and oxygen vacancies on dielectric and magnetic properties of Pb2+ ion doped LaFeO3 polycrystalline ceramics. Physica B 448:304
Phokha S, Hunpratup S, Pinitsoontorn S, Maensiri S (2015) Structure, magnetic, and dielectric properties of Ti-doped LaFeO3 ceramics synthesized by polymer pyrolysis method. Mater Res Bull 67:118
Lüning J, Nolting F, Scholl A, Ohldag H, Seo JW, Fompeyrine J, Locquet J-P, Stöhr J (2003) Determination of the antiferromagnetic spin axis in epitaxial LaFeO3 films by x-ray magnetic linear dichroism spectroscopy. Phys Rev B 67:214433
Li S, Wang X (2015) Synthesis of different morphologies lanthanum ferrite (LaFeO3) fibers via electrospinning. Optik 126:408
Xiao H, Xue C, Song P, Li J, Wang Q (2015) Preparation of porous LaFeO3 microspheres and their gas-sensing properties. Appl Surf Sci 337:65
Tang P, Tong Y, Chen H, Cao F, Pan (2013) Microwave-assisted synthesis of nanoparticulate perovskite LaFeO3 as a high active visible-light photocatalyst. Appl Phys 13:340
Sivakumar M, Gedanken A, Zhong W, Jiang YH, Du YW, Brukental I, Bhattacharya D, Yeshurunc Y, Nowikd I (2004) Sonochemical synthesis of nanocrystalline LaFeO3. J Mater Chem 14:764
Velichkova MM, Lazarova T, Tumbalev V, Ivanov G, Kovacheva D, Stefanov P, Naydenov A (2013) Complete oxidation of hydrocarbons on YFeO3 and LaFeO3 catalysts. Chem Eng J 231:236
Kansara SB, Dhruv D, Joshi Z, Pandy DD, Rayaprol S, Solanki PS, Kuberkar DG, Shah NA (2015) Structure and microstructure dependent transport and magneticproperties of sol–gel grown nanostructured La0.6Nd0.1Sr0.3MnO3 manganites: role of oxygen. Appl Surf Sci 356:1272
Liua T, Xu Y (2011) Synthesis of nanocrystalline LaFeO3 powders via glucose sol–gel route. Mater Chem Phys 129:1047
Yang Z, Huang Y, Dong B, Li HL (2006) Controlled synthesis of highly ordered LaFeO3 nanowires using a citrate-based sol–gel route. Mater Res B 41:274
Cullity BD, Stock SR (2001) Elements of X-ray Diffraction. 3rd edn, Prentice-Hall, Upper Saddle River, NJ
Majeed Khan MA, Kumar S, Ahamed M (2015) Structural, electrical and optical properties of nanocrystalline silicon thin films deposited by pulsed laser ablation. Mat Sci Semicon Proc 30:169
Cho YG, Choi KH, Kim YR, Jung JS, Lee SH (2009) Characterization and catalytic properties of surface La-rich LaFeO3 perovskite. Bull Korean Chem Soc 30:6
Lee WY, Yun HJ, Yoon JW (2014) Characterization and magnetic properties of LaFeO3 nanofibers synthesized by electrospinning. J Alloys Compd 583:320
Jaiswal A, Das R, Maity T, Vivekan K, Abraham PM, Adyanthaya S, Poddar P (2010) Temperature-dependent raman and dielectric spectroscopy of BiFeO3 nanoparticles: signatures of spin-phonon and magnetoelectric coupling. J Phys Chem C 114:2108
Schuele WJ, Deetscreek VD (1962) Appearance of a weak ferromagnetism in fine particles of antiferromagnetic materials. J Appl Phys 33:1136
Acknowledgments
This work was financially supported by the Integrated Nanotechnology Research Center (INRC), Department of Physics, Faculty of Science, Khon Kaen University, Thailand. Financial assistance also comes from the Higher Education Research Promotion and National Research University Project of Thailand, Office of the Higher Education Commission and the Nanotechnology Center (NANOTEC), NSTDA, Ministry of Science and Technology, Thailand through its program of Center of Excellence Network. Thanks are due to Rajamangala University of Technology Rattanakosin Wang Klai Kangwon Campus, Thailand (Grant A21/2559) for co-providing financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Swatsitang, E., Karaphun, A., Phokha, S. et al. Investigation of structural, morphological, optical, and magnetic properties of Sm-doped LaFeO3 nanopowders prepared by sol–gel method. J Sol-Gel Sci Technol 81, 483–492 (2017). https://doi.org/10.1007/s10971-016-4212-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-016-4212-z