Abstract
Mesoporous alumina and alumina–sodium aluminate composites were obtained by sol–gel method using polyethyleneimine as template. New approach for the regulation of the micro- and nanostructure of the composites as catalytic materials was proposed, exploiting inorganic seeds for the control of morphology for the produced nanostructures. Composition and temperature window for preventing the leaching of sodium aluminate in the course of reaction and thus drastically improving the catalytic activity has been identified. Structure and phase composition of the thus obtained catalytic materials were characterized using X-ray diffraction, N2 adsorption/desorption, FTIR spectra, scanning electron microscopy, and thermal analysis. New type of catalyst has shown high efficiency in the vegetable oil transesterification process under mild conditions opening prospects for small-scale production of reasonably good-quality biodiesel fuel.
Graphical Abstract
Dissolving sodium aluminate in the alumina matrix at proper compositions and under controlled temperatures permits to drastically decrease the leaching of aluminate and maintain its high catalytic activity in transesterification of vegetable oils with methanol.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Biodiesel has recently attracted much attention as less polluting fuel obtained from renewable resources [1]. The major challenge in its broad-scale application is cost-efficient production of the fuel with sufficient quality. General approach to biodiesel is catalytic alkylation of vegetable oils. Catalysts used in the transesterification of triglycerides are alkali, acid, enzyme or heterogeneous catalysts, among which alkali catalysts such as sodium hydroxide, sodium methoxide, potassium hydroxide, and potassium methoxide are considered more efficient [2]. In particular, the production of biodiesel from soybean oil by transesterification was carried out over sodium aluminate as heterogeneous catalyst [3]. Solid base showed high catalytic activity for methylation with methanol as reagent reaching a 93.9 % yield. As shown in [3], the aluminate has significant solubility in methanol, resulting in formation of soaps. Calcination of the aluminate decreases its solubility. However, with the increase in calcination temperatures, the basicity, solubility, and catalytic activity were also decreased. It has to be noted that solubility of sodium aluminate may also significantly increase when considerable water content is present in the methanol or in oil.
It is plausible to suppose that using a highly porous substrate, such as mesoporous matrix, for deposition of the sodium aluminate catalyst; it should be possible to maintain its high activity even when its solubility is decreased by calcination.
Mesoporous materials as a class of nanostructured materials are very perspective as catalysts and catalyst supports in heterogeneous catalysis. Mesoporous catalysts have large active surface area, good mechanical hardness, and very good hydrodynamic parameters for the contact with liquid phase [4–9]. Commonly mesoporous catalysts based on silica and even metal oxides are formed exploiting self-assembly of large organic surfactant or block copolymer molecules [4–9]. For metal oxide-based mesoporous solids an alternative to this approach are provided by self-assembly mechanisms guiding the aggregation of the primary oxometallate particles, MTSALs [10, 11]. An example of this type of approach has been described for the synthesis of γ-Al2O3 with the help of sodium salicylate as the source of small ligands for charging the surface of the primary particles [12].
The γ-Al2O3 is well known as a catalytically active material and catalyst support. Its catalytic activity is provided by the presence of Lewis and Brønsted acid and base centers [13–16]. Prospects of the future γ-Al2O3 applications are associated with its use as a biodiesel catalyst. A large number of catalytic systems for biodiesel production based on γ-Al2O3 have been studied, e.g., alkaline earth metal oxides/Al2O3, Li–CaO/Al2O3, La2O3/ZnO/Al2O3, La2O3/Al2O3, γ-Al2O3/ZrO2, KI/Al2O3, NaOH/Al2O3, KF/Al2O3, etc. [3, 17–21] However, mesoporous structures based on the γ-Al2O3–NaAlO2 system have to the best of our knowledge not been reported yet.
In our recent study [22], we showed possibility to obtain the wormlike mesoporous structure of γ-Al2O3 and of γ-Al2O3–CuO with high surface area and pore diameter of 8–12 nm, using polyethyleneimine (PEI) as template. PEI is a branched-chain water-soluble polymer which is widely used as pore-forming agent in the membrane fabrication [23]. This water-soluble polymer is defined as a pore-forming agent. The PEI has been applied as complexing template [22], which formed complexes with copper ions. It was thus possible to create high concentrations of copper ions in the mesopores of γ-Al2O3. Sol–gel process in that case was carried in acidic medium [22]. The structure of the PEI-copper complexes was proved to be linear. The difficulty and challenge in producing mesoporous γ-Al2O3–NaAlO2 catalyst using the PEI template is that PEI in alkaline medium has the form of a coil.
The purpose of this work has been to develop a new approach to the synthesis of mesoporous γ-Al2O3–NaAlO2 catalyst structures using combination of template and seed methods in sol–gel synthesis and finding the composition and temperature window for improvement of their activity through prevention of sodium leaching.
2 Experimental
All reagents, except water, were purchased from Sigma-Aldrich and used as received.
The boehmite sol was prepared by Yoldas method [24, 25] through peptization of aluminum hydroxide precipitate with nitric acid, as described in [26]. Stable boehmite sols were produced by mixing 16 g of aluminum iso-propoxide Al(OC3H7)3 with 100 ml of deionized water, followed by peptization of the resultant white precipitate with 2 ml of concentrated nitric acid at 80 °C under vigorous stirring for 2 h (solution 1). The template solution was prepared by dissolving 4 g of the branched PEI, average M w ~ 25,000 in 10 ml deionized water with stirring for 30 min at 40 °C (solution 2). Subsequently, the solution 1 was mixed at 80 °C with solution 2 under vigorous stirring and a yellowish gel was formed (solution 3). At the next step, NaAlO2 was added at 80 °C to the solution (3) in 5, 10, 20, 40, 60, and 80 % fraction of the total catalyst weight (solution 4). Solution 4 was sonicated in an ultrasonic bath and evaporated in an air thermostat at 70 °C providing a dry solid. The mesoporous product has been obtained by calcination of the organic-containing samples at 600 °C for 3 h in air.
The dried and sintered samples were characterized by XPD using Bruker D8 Advance X-ray powder diffractometer with CuKα radiation (15,478 Å), thermally with the NETZSH STA 409PC thermal analyzer, and by FTIR spectroscopy with Avatar 360 FTIR ESP instrument using KBr tablets, and by scanning electron microscopy with Hitachi TM-1000-μ-DeX instrument, and for high-resolution images, with Zeiss NVision 40 instrument. The specific surface area and pore size distribution of the catalyst samples were determined by multi-point BET and BJH methods, using N2 gas adsorption–desorption techniques (Quantachrome Nova 1200). The yield of esterification reaction was determined from quantitative 1H NMR measurements using Bruker Avance 500 MHz instrument.
3 Results and discussion
The insight into the crystal structure of the produced catalysts was provided by the XRD studies. Figure 1a shows the XRD patterns of dry composite samples, with diffraction maximums of pseudo-boehmite (AlOOH) at 2θ = 14°; 28°; 38.5°; 49°. The increase in concentration of sodium aluminate leads to more distinct reflections of this latter phase with diffraction maxima at 2θ = 18°; 21°; 29°; 32°; 36°; 39°; 40° for the as-prepared sample [27].
Calcination at 600 °C leads to high extent of amorphization—only one broad peak of boehmite is visible at 2θ = 47° (Fig. 1b). It is important to notice that the structure remains essentially amorphous independent of the calcinations time. Calcination at higher temperatures (the data for 950 °C is shown in 1d for the composite with 40 % of NaAlO2) leads to re-appearance of clearly defined crystalline phases of both boehmite and sodium aluminate. The disappearance of the separate NaAlO2 phase at intermediate temperature is most probably due to its intermediate dissolution (melting in) into the alumina phase. The morphology and porosity of the material remain unchanged at this intermediate temperature, but the leaching of NaAlO2 is drastically decreased (for details see below) which gives rise to increased and stabilized catalytic activity.
The morphological and chemical characteristics of the samples are compared in Fig. 2. General appearance of both the pure boehmite (Fig. 2a) and the composites with NaAlO2 is gel-like, while the pure NaAlO2 displays micro-crystalline powder morphology (Fig. 2f). The most interesting feature of the produced gels is their different surface compositions for samples with different sodium aluminate content: both the samples with low and with high NaAlO2 content do reveal sodium in the surface, while the intermediate content samples (40–60 % NaAlO2) contain only aluminum in the surface layer.
This can be explained through different chemical behavior of sodium aluminate seed material dependent on its content. At low content (up to 20 %), the medium is predominantly acidic and the nucleation of the γ-Al2O3 occurs independently of the seeds, making the sodium content measurable. At the intermediate fraction (40–60 %), the medium becomes basic through leaching from the aluminate phase and the boehmite nanoparticles from the sol precipitate predominantly on the grains of NaAlO2. At even higher content of the aluminate, the leaching is strong and can supposedly result in re-precipitation of NaAlO2, resulting in growth of its grains together with boehmite and again in measurable concentration of sodium on the surface.
More detailed information about the micro- and nanostructure of the samples was provided by high-resolution SEM (see Fig. 3). It is clearly visible that the material in case of the catalytically most active samples with 60–80 % content of NaAlO2 is a nanocomposite built up of larger microcrystals of γ-Al2O3 shaped as hexagonal rods and containing relatively much less sodium (below 1 wt%) together with a much more Na-rich nano powder (with single particles about 10 nm in size, forming wormhole mesoporosity via aggregation), where the phase of sodium aluminate, NaAlO2, apparently is dominating. It can be hypothesized that the improved stability of the catalysts with intermediate sodium aluminate content is due to the dissolution of NaAlO2 in mesoporous γ-Al2O3 crystals (as indicated by EDS). The basic centers are supposedly located in the pores within the alumina matrix and are rendered stable to leaching.
The information about bonding in the as-prepared and calcined γ-Al2O3–NaAlO2 samples can be obtained also from the FTIR spectra (see Fig. 4).
The spectra display peaks corresponding to stretching of the Al-O (peaks of 590 and 630 cm−1) and Al–O–Al (750 and 800 cm−1) bonds, bending vibrations of H2O molecules (1620, 1630 cm−1) and of hydroxyl—OH groups (1550 cm−1). The broad band in the area 2850–3490 cm−1 reveals existence of nonequivalent molecules of water, –OH groups, and hydrogen bonds. Peaks at 1350 and 1370 cm−1 correspond to stretching of the C–H fragments. Bands at 2900 and 2910 cm−1 reveal existence of CH2-groups which partially remain even after calcination.
The provided absorption bands can be observed in the spectra of both calcined and not calcined samples, the difference been in appearance of the Al–O–Na (1015 cm−1) bands, qualitatively proving existence of an active phase in not calcined samples.
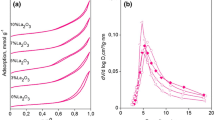
The porosity and surface area in the heat-treated samples were investigated by nitrogen sorption experiments (see Fig. 5). In all cases, it is apparent that the powders exhibit classical type-IV isotherm curves with a hysteresis loop, according to the IUPAC classification [28]. The active surface area and porosity of the pure γ-Al2O3 samples was quite resemblant of the values reported in [12] for self-assembled mesoporous alumina.
As it can be seen in Fig. 5, the content of NaAlO2 in composites affects significantly the structure of resulting materials. With increasing NaAlO2 concentration up to 40 %, a displacement of the start of the hysteresis loops along the P/P0 axis from 0.57 to 0.7 is observed. Further increase in the content of NaAlO2 leads to reverse in the displacement of hysteresis loops that also change form. The pores of slits type presumably have been formed instead of wormlike cylindrical pores in the materials (for BET and BJH pore size distribution, please, see Table 1; Fig. 6).
When the dry samples with 5 % NaAlO2 were calcined at 600 °C for 2 h, the highest specific surface area, 262 m2/g, was obtained with the corresponding pore volume of 0.48 cm3/g and average pore size of 7.4 nm.
The increase in the NaAlO2 content from 5 to 80 % is associated with decrease in the specific surface area from 262 m2/g to 91 m2/g, and the pore volume decrease from 0.48 to 0.19 cm3/g, but the pore diameter increases at the same time from 7.4 to 20 nm. This effect can be ascribed to the state of the polyethyleneimine in the composites with different contents of NaAlO2 and to the structural changes on the polyethyleneimine removal.
Thermogravimetric and differential thermal analyses of as-sintered dry Al2O3–NaAlO2 samples containing 40, 60, and 80 wt% NaAlO2 were performed at the heating rate of 5 °C/min (see Fig. 7). The thermal transition of boehmite to γ-Al2O3 [29] has been associated with the following effects in the DTA curve: (1) loss of physically bound water—a peak near 90 °C; (2) loss of chemically bound water—a peak near 200 °C; (3) transformation of boehmite into γ-Al2O3 (or transitional form)—the peak near 380 °C; and (4) dehydration of the residual hydroxide groups finishing with crystallization of the alpha alumina. The last step gives no thermal event, but appears as a continuous mass loss and seems to stop at about 900 °C.
In the Fig. 7, the first endothermic peak in the DSC curve is associated with the loss of physically bound water. For the NaAlO2 concentration of 40, 60, and 80 %, the DSC curves display double peaks with maxima at 200 and 300 °C. The first peak can be associated with the loss of chemically bound water. The second peak is corresponding to the thermal degradation of the PEI molecules adsorbed on the outer surface of the inorganic particles. Small exothermic peak with a maximum near 400 °C may be associated with the transition of boehmite into the γ-Al2O3. Exothermic peak at 500 °C can be attributed to the combustion of polyethyleneimine, entrapped in the matrix of aluminum oxide. This peak is revealed only for the compositions that contain 40 and 60 % of NaAlO2. This may indicate different mechanisms of action of template in these systems. At the NaAlO2 content of 40 and 60 %, large portions of PEI were included in the inorganic matrix. With 80 % content of NaAlO2, all PEI molecules are on the surface of the inorganic particles and are not involved in the formation of mesophases.
Commercial edible-grade canola oil as the source for catalytic tests in biodiesel production was obtained from the market. The transesterification process has been performed in a 10 cm3 sealed glass bottle with the oil/methanol = 1:5 molar ratio, and 0.2 g of catalyst at 60 °C. The water content in methanol was 1 %. The mixture was kept under stirring for 0.5, 2, 4, and 6 h. After the reaction, the solid catalyst was separated by filtration. The liquid was put into a graded glass tube and was kept at ambient temperature for 24 h, which resulted in two liquid phases. The upper layer was biodiesel, and the lower layer was glycerin. The transesterification products were analyzed according to [30–33]. The biodiesel was characterized by 1H NMR spectroscopy, and its spectrum in CDCl3 is shown in Figure FS 1. The characteristic peak of methoxy protons was observed at 3.64 ppm and of the α-CH2 protons at 2.288 ppm. 1H NMR has been used to quantify the conversion of vegetable oil to methyl esters by transesterification reaction. The equation used to quantify the yield of transesterification was according to [32, 33]:
where C is percentage conversion of triglycerides to corresponding methyl esters; AMe is peak area for the methoxy protons of the methyl esters, and ACH2 is the peak area for carbonyl methylene protons (see Fig. FS1).
The best catalytic activities were displayed by the catalysts with 40 and 60 % of NaAlO2. In the oil–methanol system using the catalyst with 80 % NaAlO2, the soap formed as major product of the reaction. Oil–biodiesel conversion using a catalyst containing 60 % of the NaAlO2 reached 60 % in 30 min and increased to about 85.7 % within 2 h (see Fig. 8).
The FTIR spectra can be used to estimate the quality of the biodiesel from transesterification of canola oil (Fig. FS2). The strong ester peaks at 1750 cm−1 (C=O ester) and at 1170–1200 cm−1 (C–O ester) are clearly present in the spectra together with the characteristic peak of the CH3-group in the methyl ester at 1445 cm−1. FTIR spectra of pure glycerin from Sigma-Aldrich and glycerin as produced in the transesterification process correlate well with each other. The selectivity of transformation into methyl esters is at 60 °C about 64 % and increases rapidly with the increase in temperature making the process attractive for the small-scale production of biodiesel.
4 Conclusions
Mesoporous alumina–sodium aluminate composite materials have been successfully produced by templating-and-seeding sol–gel method. The sodium aluminate micro powder was introduced directly into the sol of aluminum oxide and acted as seeding material. Depending on the synthesis conditions and the content of sodium aluminate, the materials with different active surface area (from 91 to 262 m2/g), pore size (from 7 to 20 nm), and pore volume (from 0.19 to 0.48 cm3/g) were produced. The freshly prepared material has according to X-ray diffraction predominantly the pseudo-boehmite structure that transforms into γ-Al2O3 on the heat treatment required for the template removal. The thermal window for dissolution of sodium aluminate in the alumina matrix permitting to drastically decrease the leaching of aluminate and maintain its high catalytic activity has been identified. The resulting material revealed high catalytic activity in transesterification of vegetable oils with methanol. The conversion of oil into methyl esters of fatty acids reached 85.7 % after 2 h at 60 °C. This simple approach opens prospects for small-scale production of reasonably good-quality biodiesel fuel.
References
Balat M, Balat H (2008) Energy Convers Manag 49:2727–2741
Meher LC, Sagar DV, Naik SN (2006) Renew Sustain Energy Rev 10:248–268
Wan T, Yu P, Wang S, Luo Y (2009) Energy Fuels 23:1089–1092
Frost R, Zhu HY, Wu P, Bostrom T (2005) Mater Lett 59:2238–2241
Kresge CT, Leonowicz ME, Roth WJ, Vartuli JC, Beck JS (1992) Nature 359:710–712
Bagshaw SA, Prouzet E, Pinnavaia TJ (1995) Science 269:1242–1244
Corma A (1997) Chem Rev 97:2373–2419
Taguchi A, Schuth F (2005) Micropor Mesopor Mater 77:1–45
Cejka J (2003) Appl Catal A Gen 254:327–338
Kessler VG, Spijksma GI, Seisenbaeva GA, Håkansson S, Blank DHA, Bouwmeester HJM (2006) J Sol Gel Sci Technol 40:163–179
Seisenbaeva GA, Kessler VG (2014) Nanoscale 6:6229–6244
Patra AK, Dutta A, Bhaumik A (2012) J Hazard Mater 201–202:170–177
Trimm DL, Stanislaus A (1986) Appl Catal 21:215–238
Yada M, Machida M, Kijima T (1996) Chem Comm 769–770
Yada M, Kitamura H, Machida M, Kijima T (1997) Langmuir 13:5252–5257
Yada M, Hiyoshi H, Ohe K, Machida M, Kijima T (1997) Inorg Chem 36:5565–5569
Peng-Lim B, Gaanty PM, Shafida AH (2011) Chem Eng J 168:15–22
Watkins RS, Adam FL, Wilson K (2004) Green Chem 6:335–341
Chai F, Cao F, Zhai F, Chen Y, Wang X, Su Z (2007) Adv Synth Catal 349:1057–1065
Boz N, Kara M (2008) Chem Eng Comm 196:80–92
Refaat AA (2011) Int J Environ Sci Tech 8:203–221
Vinogradov VV, Agafonov AV, Vinogradov AV, Guliaeva TI, Drozdov VA, Licholobov VA (2010) J Sol Gel Sci Technol 56:333–339
Xiang T, Zhao L, Li Y, Lei Z, Jin S, Li S, Li Y, Liang Y (2008) Mater Lett 62:1627–1629
Yoldas BE (1975) Am Ceram Soc Bull 54:286–288
Yoldas BE (1973) J Appl Chem Biotechnol 23:803–809
Pai RV, Pillai KT, Pathak S, Mukerjee SK, Vinogradov VV, Agafonov AV, Vinogradov AV, Aggarwal SK (2012) J Sol Gel Sci Tech 61:192–196
Rodgers KA, Gregory MR, Barton R (1991) Clays Clay Miner 39:103–107
Kaneko K (1994) J Membr Sci 96:59–89
Alphonse P, Courty M (2005) Thermochim Acta 425:75–89
Mahamuni NN, Adewuyi Y (2009) Energy Fuels 23:3773–3782
Tariq M, Ali S, Ahmad F, Ahmad M, Zafar M, Khalid N, Ajab Khan M (2011) Fuel Proc Tech 92:336–341
Gelbard G, Bres O, Vargas RM, Vielfaure F, Schuchardt UF (1995) J Am Oil Chem Soc 72:1239–1241
Knothe G (2000) J Am Oil Chem Soc 77:489–493
Acknowledgments
The authors express their gratitude to the Russian Ministry of Higher Education and Science for the visiting scientist at ISC RAS grant to Vadim Kessler.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Agafonov, A.V., Yamanovskaya, I.A., Ivanov, V.K. et al. Controlling micro- and nanostructure and activity of the NaAlO2 biodiesel transesterification catalyst by its dissolution in a mesoporous γ-Al2O3-matrix. J Sol-Gel Sci Technol 76, 90–97 (2015). https://doi.org/10.1007/s10971-015-3755-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-015-3755-8