Abstract
In this work, we report the application of a sol–gel derived ZnO thin film as a buffer layer for high efficiency inverted polymer solar cells. ZnO films are widely used in such devices because they have a relatively high electron mobility, high transparency and environmental stability. The ZnO precursor was prepared by dissolving zinc acetate and ethanolamine in the 2-methoxyethanol. ZnO thin films were then deposited on indium tin oxide (ITO)/glass substrates by spin coating the above solution. Inverted polymer solar cells with the configuration ITO/ZnO/photoactive layer/MoOx/Ag were realized in order to investigate the performance of ZnO thin film. The photoactive layer is a blend of poly[(4,8-bis-(2-ethylhexyloxy)-benzo[1,2-b;4,5-b′]dithiophene)-2,6-diyl-alt-(4-(2-ethylhexanoyl)-thieno[3,4-b]thiophene)-2,6-diyl] (PBDTTT-C) and [6,6]-phenyl C71 butyric acid methyl ester ([70]PCBM) (1:1.5 w/w). We made a comparative study of the photovoltaic behavior of devices with ZnO films deposited using different sol–gel recipes. In particular, we varied the zinc acetate/ethanolamine molar ratio to have ZnO films with different trace amounts of starting materials. In addition we also prepared ZnO films annealed at 200 °C for different times (from 5′ to 60′) in order to evaluate this effect on the trace amount removal.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
A promising alternative to silicon-based devices, for the production of clean and renewable energy, is recognized by polymer solar cells (PSCs) [1]. The low-cost, the high processability using roll-to-toll methods and the flexibility are the main advantages of this novel technology [1–3]. Actually, the most successful device architecture is based on the bulk heterojunction configuration, where a conjugated polymer (electron donor) is intimately mixed with a fullerene derivative (electron acceptor) giving a nanoscale interpenetrating network with phase-separated domains [4, 5]. The major challenges in the commercialization of PSCs are the competitive power conversion efficiency (PCE) and the long-term air stability. Recently, PCE up to 9 % has been reached using low-bandgap polymers [6].
In the conventional device structure of the PSCs, a transparent conductive anode [e.g. indium tin oxide (ITO)] is typically covered by a thin hole conducting layer, such as poly(3,4-ethylenedioxythiophene)-poly(styrenesulfonate) (PEDOT:PSS), over which, the active layer is deposited; the device is completed with a low-work-function metal (usually Ca/Al) [1]. This configuration has limited device stability because the cathode is susceptible to degradation by oxygen and water vapor [7] and PEDOT:PSS is hygroscopic and acidic producing fast degradation of ITO [8, 9].
In order to avoid these issues and improve the stability of the PSCs, the inverted configuration has been developed [10, 11]. In this architecture, the charge-collecting nature of the electrodes is reversed: ITO electrode, covered by a n-type metal oxide such as titanium oxide or zinc oxide, is used as the cathode and an air-stable high-work-function metal (e.g. Ag) is used as the anode. Such structure doesn’t suffer from the top metal contact oxidation and ITO/PEDOT:PSS interface stability.
ZnO is an interesting electron transport layer (ETL) for inverted PSCs due to its high transparency in the visible range, relatively high electron mobility and environmental stability. Since the valence band of ZnO is much lower than those of the highest occupied molecular orbital of the donor and acceptor materials usually employed in the realization of the blend, it also acts as hole blocking layer. In addition, the possibility to be easily processed via a solution method followed by low temperature annealing, makes ZnO fully compatible with flexible substrates using roll-to-roll methods [1].
Trace amount of the precursor materials used to prepare the starting solution, can be still present in the deposited ZnO film. The contaminants can have a great impact on the quality of the ZnO layer and can dramatically influence the performance of the devices [12]. To the best of our knowledge, this issue was only studied for PSCs based on a blend of poly-3-hexylthiophene (P3HT) and [6,6]-phenyl C61 butyric acid methyl ester ([60]PCBM), that requires a post deposition annealing [12], which can contribute to further modify the ZnO surface, but there is no systematic study in the literature on the impact on blend materials that don’t need additional thermal treatments.
In this paper we realized high efficiency inverted PSCs with a sol–gel derived ZnO thin film (starting from a solution of zinc acetate and ethanolamine in 2-methoxyethanol) as an electron transport layer. The device architecture was glass/ITO/ZnO/photoactive layer/MoOx/Ag. The photoactive layer is a blend of poly[(4,8-bis-(2-ethylhexyloxy)-benzo[1,2-b;4,5-b′]dithiophene)-2,6-diyl-alt-(4-(2-ethylhexanoyl)-thieno[3,4-b]thiophene)-2,6-diyl] (PBDTTT-C) and [6,6]-phenyl C71 butyric acid methyl ester ([70]PCBM) (1:1.5 w/w). PBDTTT-C is a copolymer of benzodithiophene and thieno-thiophene units with very promising photovoltaic properties. PCE up to 6 % has been reached when this polymer is used as donor material in conventional PSC [13]. Photovoltaic devices were realized and characterized by external quantum efficiency (EQE) and current–voltage (IV) measurements under different illumination levels. We investigated the influence of the ZnO thermal annealing time and the presence of residual organics on the photovoltaic performance of the devices.
2 Experimental part
2.1 Polymer solar cells realization
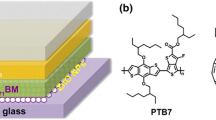
The configuration of the device structure was ITO/ZnO/PBDTTT-C:[70]PCBM/MoOx/Ag and it is shown in Fig. 1 together with the chemical structure of the materials used to prepare the photoactive layer.
The ITO coated Corning® Eagle XG glass substrates, were purchased from Delta Technologies, LTD, with a sheet resistance of 10 Ω/square. PBDTTT-C and [70]PCBM were purchased from Solarmer and Solenne BV, respectively, and used as received.
The ZnO precursor was prepared by dissolving zinc acetate dihydrate (ZnAc, Zn(CH3COO)2·2H2O, Aldrich, 99.9 %, 1 g) and ethanolamine (MEA, NH2CH2CH2OH, Aldrich, 99.5 %, 0.28 g) in 2-methoxyethanol (CH3OCH2CH2OH, Aldrich, 99.8 %, 10 ml). The solution was vigorous stirred for 18 h in air for the hydrolysis reaction [14–16]. We also prepared two other solutions containing the same amount of zinc acetate but 0.14 and 0.56 g of ethanolamine in order to have a molar ratio between the zinc precursor and the stabilizer 1:0.5 and 1:2 (the standard one is 1:1).
The ZnO films were realized by spin coating the above solutions on the top of glass/ITO patterned substrates. The films obtained from the 1:1 solution of ZnAc and MEA were annealed at 200 °C for different annealing time (from 5′ to 60′). The films obtained from other molar ratios between ZnAc and MEA were annealed at 200 °C for 60′. The thickness of the ZnO film was 40 nm.
The active layer (80 nm) was prepared by dissolving PBDTTT-C (15 mg/ml) and [70]PCBM (22.5 mg/ml) in a mixed solvent of 1,2-dichlorobenzene/1,8-diiodoctane (97:3 % by volume) and spin coated on the top of ZnO film. A 5 nm MoOx layer and a 100 nm Ag layer were subsequently thermally evaporated through a shadow mask to form a top anode. The active area of the device is 20 mm2.
2.2 Materials and device characterization
The film thicknesses and the roughness were measured by KLA Tencor P-10 surface profiler. UV–Vis optical transmittance analysis of layers deposited on glass substrates was carried out using a Perkin-Elmer Lambda 900 spectrophotometer. The surface morphology of ZnO films was analyzed by scanning electron microscopy (SEM). X-ray diffraction measurements were carried out by X’PERT-MPD (Philips) diffractometer using a Cu Kα radiation source. The samples was prepared by spinning the solution containing ZnAc and MEA (1:1) on a silicon (100) wafer at 4,000 rpm and then annealed at 200 °C for 10′; this procedure was repeated five times in order to have a thicker sample. Fourier transform infrared (FTIR) spectra have been recorded on a Bruker Fourier transform spectrometer model Equinox55, equipped with a deuterated triglycine sulphate detector operating in the range 400–4,000 cm−1. The ZnO samples were prepared by spinning solution on a silicon (111) wafer at 4,000 rpm and annealed at 200 °C.
All the PSCs were characterized in air and without encapsulation by means of EQE and IV measurements performed under simulated AM 1.5G illumination. IV light characteristic was measured with a Keithley 2,400 source measure unit (Keithley Instruments Inc., Cleveland, USA). The voltage ramp rate, controlled by a PC program, was of 10 mV/s from negative to positive potential. Simulated AM 1.5G white light illumination was provided by a class “AAA” Photo Emission Tech solar simulator, Model CT100AAA, equipped with a 150 W Xenon lamp and its intensity was calibrated using a mono-Si reference cell equipped with a KG5 filter for 1 sunlight intensity of 100 mW/cm2. All the photovoltaic properties were recorded in ambient air at room temperature (~25 °C).
3 Results and discussion
ZnO was synthetized by sol–gel from zinc acetate dihydrate and ethanolamine (used as stabilizer) in 2-methoxyethanol. Figure 2 shows the transmittance spectrum of a film of ZnO deposited on a glass substrate by spin coating a solution containing an equimolar amount of ZnAc and MEA and annealed at 200 °C for 60′. The thickness is around 40 nm. The layer is transparent from 400 nm (transmittance greater than 90 %, very close to the one of the pristine glass substrate) and it only blocks the light at shorter wavelengths. The optical properties of ZnO are suitable for the absorption of the used blend (PBDTT-C:[70]PCBM) which shows a good spectral response in the range 400–700 nm. Since the incoming light has to cross the ZnO film in the inverted architecture, the transparency is crucial when this ETL is used as a front buffer layer.
In Fig. 3 we report the X-ray diffraction pattern of a ZnO film. For this analysis, the sample was obtained by spinning the solution containing ZnAc and MEA (1:1) on a silicon (100) wafer at 4,000 rpm and then annealed at 200 °C for 10′. This procedure was repeated five times in order to have a thicker sample. The X-ray diffraction profile shows a broad peak in the 2θ range 32°–36° due to the overlapping of the amorphous phase of ZnO with the (100), (002) and (101) reflections of ZnO (Joint Committee on Powder Diffraction Standards no. 36-1451) which are a characteristic signature of nanocrystals of ZnO. Instead, sharp peaks are typically observed for highly crystalline ZnO films obtained by annealing at temperature greater than 400 °C [17–19]. It is worth to note that the ZnO sample realized for X-ray analysis is different from the one used to prepare the ETL in the PSCs: in fact, the ZnO is deposited on a silicon substrate instead of glass/ITO and the thickness is greater. For this reason, it is not possible to make considerations about the presence of nanocrystals in the films used as ETL for the realization of PSCs.
In Fig. 4 we report the SEM micrographs of a ZnO sample deposited on glass substrate [by spinning the solution containing ZnAc and MEA (1:1)] and annealed at 200 °C for 60′. The film seems very uniform with low roughness (around 5 nm as measured from mechanical profilometer).
In Fig. 5 we report the FTIR spectra of films of ZnO deposited on a silicon (111) substrate (single deposition) starting from an equimolar solution of ZnAc and MEA. These films were annealed at 200 °C for 5′ and 60′. We can observe the presence of two broad peaks at 1,590 and 1,425 cm−1. The first one corresponds to the asymmetric stretching vibration of the C=O group of the zinc acetate, while the second one is the superposition of the symmetric stretching vibration of the same C=O group with the stretching vibration of the N–H group of the amine (1,420 cm−1) [20, 21]. The annealing at 200 °C, which is the temperature commonly used to post process the ZnO fresh deposited film in order to use it as ETL in PSCs, is not able to remove completely the traces of the precursors although the annealing time has a great impact as we can observe from Fig. 5. In fact, zinc acetate is a salt while ethanolamine is a liquid with 170 °C boiling point. It has been shown that the presence of ethanolamine on the top of ZnO thin films significantly decreases the photovoltaic performance of inverted devices based on a blend film of P3HT and [60]PCBM [12]. When P3HT:[60]PCBM is deposited on top of ZnO surface and it is annealed (process requested in order to have the right morphology of this material), there is the formation of [60]PCBM clusters that alter dramatically the morphology of the photoactive film producing poor performance of the devices [12].
Recently, copolymers of benzodithiophene and thieno-thiophene units exhibited very promising properties as donor materials in PSCs due to their relatively large and planar conjugated structure, which can promote facial π–π stacking of the molecules increasing the charge transport and the red shift of the absorption spectra [22, 23]. These materials are usually used as blend with [70]PCBM and don’t need post processing annealing. In this case, thought the fullerene cluster formation is not a critical issue for the blend morphology, the traces of ZnAc and MEA can still influence the recombination processes at the interface.
In order to understand the impact of trace amounts of ZnAc and MEA on the performance of devices realized using low-bandgap polymers that don’t require post processing annealing, we studied the impact of ZnO films deposited using different sol–gel recipes on the photovoltaic parameters of PSCs. We varied the ZnAc:MEA molar ratio to obtain ZnO films with an excess or deficiency of the stabilizer. In addition, we also prepared different ZnO films treated at 200 °C for different time (from 5′ to 60′) with the aim to evaluate the effect of the annealing time on the trace amount removal.
We tested the performance of the ZnO thin films as ETL in inverted devices with the following architecture: glass/ITO/ZnO/blend/MoOx/Ag. The photoactive layer is a blend film of PBDTTT-C:[70]PCBM (1:1.5 w/w) and it was prepared by spin-coating as reported in the experimental part. All the devices were prepared under an inert nitrogen atmosphere and characterized in air, without encapsulation, by EQE and IV measurements performed under simulated AM 1.5 G illumination (100 mW/cm2).
We prepared three different ZnO films starting from solutions containing ZnAc and MEA with the molar ratios 1:1, 1:0.5 and 1:2 (i.e. solutions with stoichiometric amount of the reactants or with an excess of ZnAc or of MEA) as described in the experimental part. All the films were annealed at 200 °C before spinning the blend solution of PBDTT-C:[70]PCBM. This temperature has been reported as the best condition to anneal the ZnO layer to be used as ETL in PSCs [16].
The IV light curves of the corresponding devices are reported in Fig. 6. Table 1 summarizes the extracted photovoltaic parameters. The best PCE was obtained for the device based on ZnO film with a ZnAc:MEA 1:1 ratio (6.35 %). Starting from a sol–gel solution with an excess of ZnAc or MEA, the highest PCEs were 6.28 and 6.08 %, respectively. The fill factor (FF) of all the devices was very similar (less of 1 % variation), namely 59 %. The short circuit current density (Jsc) is greater for the device with ZnO obtained from a solution with a balanced molar ratio (1:1) of the ZnO precursor and the amine. Since the collection of electrons to the ITO cathode is enhanced by better reduction of the bulk electric field, it means that the “cleaner” ZnO film shows a better hole blocking capability. The transmission spectra of these samples are equal, i.e. the amount of light that reaches the photoactive material is the same for all the three devices: this means that the variation of Jsc can be only ascribed to the better electrical properties of the ZnO film with less reactants traces. The open circuit voltage (Voc) is greater (711 mV) for the device using ZnO obtained from ZnAc:MEA 1:1 solution. The excess of MEA decreases this parameter to 699 mV, while in case of the surplus of ZnAc the Voc is 705 mV. Since the Voc also depends from the recombination processes at the interface between ZnO and the photoactive blend, it is obvious that a “cleaner” ZnO film produces devices with better Voc. Nevertheless, the excess of MEA is more detrimental for the performance of the devices that the excess of ZnAc, although this effect is less than 5 % of relative variation. This means that an unbalanced ratio between the starting component of the ZnO solution is not a critical issue in PBDTTT-C:[70]PCBM based devices as reported elsewhere for the P3HT:[60]PCBM ones [12].
In order to evaluate the effect of the annealing time on the performance of the cells, we realized PSCs incorporating ZnO films annealed at 200 °C with different times (5′, 15′, 30′ and 60′). The IV light curves of the corresponding devices are reported in Fig. 7. Table 2 summarizes the extracted photovoltaic parameters. The PCE of the devices decreases as the annealing time increases: it reaches the value of 6.60 % for the solar cell using a ZnO layer annealed for 5′ and 6.16 % for the one built with a ZnO layer treated for 60′. This change is mainly due to the variation of FF. In fact, the FF also decreases with the annealing time of ZnO, from 60 % for the 5′ treated film to 57.1 % for the 60′ one. The Jsc is almost constant for all the devices with only small variations (<2 %). The Voc slightly decreases upon ZnO annealing time (from 714 to 705 mV). Since the increasing annealing time should contribute to remove MEA from the ZnO film, it is expected a better Voc for the devices based on a ZnO buffer layer annealed for a longer time. Nevertheless, the series resistance of the devices increased upon annealing time: this is due to the slight increase of the sheet resistance of the ITO layer. This effect probably hides the variation due to MEA traces.
In Fig. 8, we report the EQE of the best realized device (i.e. the one with the ZnO layer deposited from an equimolar solution of ZnAc and MEA and treated at 200 °C for 5′). The device shows a broad response range from 300 to 900 nm (according to the one of the conventional device reported in the literature [13] ), with values greater than 65 % in the range 500–700 nm. The Jsc calculated from the EQE is in good agreement with the one measure from the IV-light curve.
4 Conclusions
In this work, we reported the application of a sol–gel derived ZnO thin film as a buffer layer for high efficiency inverted PSCs based on a blend film of PBDTTT-C:[70]PCBM. The ZnO precursor was prepared by dissolving zinc acetate and ethanolamine in the 2-methoxyethanol and the solution was spin coated on the top of a glass/ITO substrate. We realized PSCs with the configuration ITO/ZnO/photoactive layer/MoOx/Ag in order to investigate the impact of ZnO films deposited using different sol–gel recipes. In particular, we varied the zinc acetate/ethanolamine molar ratio to have ZnO films with different trace amounts of starting materials. We found that an excess of ethanolamine reduces the Voc and the PCE of the corresponding PSCs, but this reduction is not so dramatic like elsewhere showed for P3HT:[60]PCBM based devices [12]. In addition we also prepared ZnO films annealed at 200 °C but for different time (from 5′ to 60′). The PCE decreases upon ZnO annealing time due to the slight increasing of the sheet resistance of the ITO layer after the annealing.
References
Krebs FC (2010) Polymeric solar cells: materials, design, manufacture. DEStech Publications, Inc., Lancaster, PA, USA
Krebs FC, Tromholt T, Jørgensen M (2010) Nanoscale 2:873–886
Krebs FC, Fyenbo J, Jorgensen M (2010) J Mater Chem 20:8994–9001
Yu G, Heeger AJ (1995) J Appl Phys 78:4510–4515
Yu G, Gao J, Hummelen JC, Wudl F, Heeger AJ (1995) Science 270:1789–1791
He Z, Zhong C, Su S, Xu M, Wu H, Cao Y (2012) Nat Photon 6:591–595
Jorgensen M, Carlè JE, Sondergaard RR, Lauritzen M, Dagnaes Hansen NA, Byskov SL, Andersen TR, Larsen-Olsen TT, B¨ottiger AP, Andreasen B, Fu L, Zuo L, Liu Y, Bundgaard E, Zhan X, Chen H, Krebs FC (2013) Sol Energy Mater Sol Cells 119:84–93
Kim YH, Lee SH, Noh J, Han SH (2006) Thin Solid Films 510:305–310
De Jong MP, van Ijzendoorn LJ, de Voigt MJA (2000) Appl Phys Lett 77:2255–2257
Arias AC, Granstrom M, Thomas DS, Petritsch K, Friend RH (1999) Phys Rev B 60:1854–1860
Yuan J, Huang X, Dong H, Lu J, Yang T, Li Y, Gallagher A, Ma W (2013) Org Electron 14:635–643
Barrera D, Lee YJ, Hsu JWP (2014) Sol Energy Mater Sol Cells 125:27–32
Chen HY, Hou J, Zhang S, Liang Y, Yang G, Yang Y, Yu L, Wu Y, Li G (2009) Nat Photon 3:649–653
Yang T, Cai W, Qin D, Wang E, Lan L, Gong X, Peng J, Cao Y (2010) J Phys Chem C 114:6849–6853
Yang T, Cai W, Qin D, Wang E, Lan L, Gong X, Peng J, Cao Y (2010) J Phys Chem C 114:6849–6853
Sun Y, Seo JH, Takacs CJ, Seifter J, Heeger AJ (2011) Adv Mater 23:1679–1686
Ong BS, Li CS, Li YN, Wu YL, Loutfy R (2007) J Am Chem Soc 129:27–32
Fortunato E, Barquinha P, Pimentel A, Goncalves A, Marques A, Pereira L, Martins R (2005) Thin Solid Films 487:102–106
Znaidi L (2003) Soler Illia GJAA, Benyahia S, Sanchez C, Kanaev AV. Thin Solid Films 428:257–262
Kamalasanan MN, Chandra S (1996) Thin Solid Films 288:112–115
Bandyopadhyay S, Paul GK, Roy R, Sen SK, Sen S (2002) Mater Chem Phys 74:83–91
Chen HY, Hou J, Zhang S, Liang Y, Yang G, Yang Y, Yu L, Wu Y, Li G (2009) Nat Photon 3:649–653
He Z, Zhong C, Huang X, Wong WY, Wu H, Chen L, Su S, Cao Y (2011) Adv Mater 23:4636–4643
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Morvillo, P., Diana, R., Ricciardi, R. et al. High efficiency inverted polymer solar cells with solution-processed ZnO buffer layer. J Sol-Gel Sci Technol 73, 550–556 (2015). https://doi.org/10.1007/s10971-014-3514-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-014-3514-2