Abstract
Modification of layered clays in view to develop porous materials, mainly for catalytic applications, has been afforded in the past via intercalation reaction of aluminum and other polyoxycations or through generation of mesoporous silica between the layers of the silicate. In this paper it is introduced examples of an alternative route for the preparation of porous nanoarchitectures based on the sol–gel method that profits from the swelling ability of organoclays in organic solvents to incorporate silicon and/or other metal (e.g., Ti, Al,…) alkoxides in the interlayer region of the silicates where they are hydrolyzed in a controlled manner. Their further polycondensation originates the formation of an oxide matrix and after a thermal treatment is possible the consolidation of oxide nanoparticles between delaminated smectites and vermiculites. It is also showed how this colloidal route can be applied to the generation of oxide nanoparticles bonded to the external surface of fibrous clays, such as sepiolite. Finally, it is also summarized with various examples the potential interest of the resulting porous clay nanoarchitecture materials in applications as acid catalysts, photocatalysts or nanofillers in polymer–clay nanocomposites.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Conventional architectures based on microcrystalline porous structures such as zeolites and related solids, MOFs, as well as periodically ordered mesoporous solids (e.g., MCM and SBA materials) receive extraordinary attention since the last decades mainly because they are materials provided of high specific surface area and porosity which is therefore relevant in view to important applications [1–5]. Most of these materials presenting a three-dimensional organization are prepared mainly from silica sources that polymerize and organize their growth around organic precursors known as templates or better as structure directing agents (SDA), which are usually removed by combustion after the synthesis process [1–3, 6]. Although less known, in recent years it has been developed a group of new porous architectures that not necessarily require the use of SDA agents but uses alkoxysilanes and other typical alkoxides involved in sol–gel synthesis. In this way, the alkoxides are hydrolyzed and polycondensated in the presence of certain two-dimensionally (2D) organized solids such as swelling phyllosilicates belonging to the clay minerals family, affording the preparation of porous solids that also show elevated specific surface area, porosity and other interesting surface properties. The key of this methodology profits from the fact that these layered solids may lead to processes of intercalation and exfoliation/delamination facilitating the formation of porous solids.
Characteristic examples of this group of 2D solids able to generate these porous heterostructures are the 2:1 charged phyllosilicates belonging to the smectite clays group, such as montmorillonite, hectorite, saponite and beidellite [7] These clays are able to intercalate organic or inorganic species which act as pillars producing a permanent and stable separation between the silicate layers with galleries that allow molecular access inside the resulting materials. These porous solids have received the name of pillared clays (PILCs) and have been intensively studied in the last two decades, being specially relevant for their properties those related to the interlamellar insertion of alumina species [6, 8–11]. Along this work we will make reference to two type of natural clays, in one case showing layered structure, such as the 2:1 charged phyllosilicates named as montmorillonite and vermiculite, as well as to the microfibrous magnesium silicate known as sepiolite (Fig. 1). Usually montmorillonites are present as plate-like particles of dimensions in the order of 1–2 μm diameter and vermiculites show bigger particle size that can even reach several centimeters. A common characteristic of montmorillonite and vermiculite layered silicates is the presence of negatively charged layers, which are compensated by exchangeable cations in their interlayer space (Fig. 1a). These cations can be easily exchanged with other positively charged species including organic cations such as long-chain alkylammonium species giving rise to organo-clays [12–14]. or inorganic (poly)cations giving rise to PILCs [8–11]. The penetration ability of ions and molecules in the interlayer space could produce a separation between consecutive layers with an increase of the basal spacing known as swelling ability and giving rise to intercalation compounds [7, 13, 15]. In the case of sepiolite, a hydrated magnesium tectosilicate constituted by needle-like particles of 2–10 μm length exhibiting elevated specific surface area and low ion-exchange capacity, the crystalline structure shows an alternation of blocks and tunnels (Fig. 1b). These last intracrystalline cavities are micropores of nanometric dimensions only accessible to water and other small molecules contrarily to layered silicates in which is possible the penetration of bigger molecular species in their intracrystalline spaces. Interestingly, sepiolite exhibits free Si–OH silanol groups along the edges of the channels at the external surface of the silicate fiber, which represent good anchor points for the immobilization of diverse entities as they are susceptible to both hydrogen bonding with molecules and polymers [16] or to remain attached to diverse species by grafting reactions [17].
Schematic representation of the structure of a montmorillonite and vermiculite charged layered clay (e.g., montmorillonite) with its hydrated interlayer cations (exchangeable cations), and b sepiolite fibrous clay with the position of external silanol groups. TS: tetrahedral sheet; OS: octahedral sheet
Novel nanoarchitectures based on porous clays combining nanoparticulated solids, such as silica, alumina, titania, iron oxide (magnetite) and carbon, have been recently synthesized in our laboratory [18–27]. Among this class of heterostructures we have developed a procedure of delamination of smectites and vermiculites previously exchanged with alkylammonium ions and swollen in an appropriate solvent that are followed of a treatment with alkoxides in which through sol–gel processes diverse type of silica or metal oxide networks are developed within the intracrystalline space of the 2D silicates [18–22]. A similar colloidal procedure can be also applied to sepiolite and palygorskite modified with different type of surfactants representing a new way for the preparation of new porous nanoarchitectures consisting in silica, alumina, silico–alumina and titania nanoparticles assembled to these fibrous silicates [23, 26, 27]. Potential applications of the resulting porous nanoarchitectures based on layered and fibrous clays modified by combination with the abovementioned nanoparticles following sol–gel procedures, include their use as adsorbents, catalysts, fillers for polymers, membranes and active phase of sensors.
2 Intracrystalline access and delamination of clays using alkoxides
As above indicated, layered clays are 2D solids that can be regarded as materials with controllable porosity because they are potentially able to be expanded by intercalation offering their internal crystal surfaces (interlamellar space) to diverse guest species (ions, discrete molecules, polymers). In fact, these silicates have a significant extent of their intracrystalline surface area with calculated values of around 750 m2/g [28]. From a theoretical point of view, these maximum values of the surface area would be attained by a hypothetical whole separation of the silicate layers, i.e., through a full delamination process. The formation of PILCs, reported in the two last decades, drives to a significant increase in the available surface area of the clay. On the other hand, delamination of the silicate in the intercalation of polymers, i.e., forming the so-called polymer–clay nanocomposites [13, 29, 30], does not lead to porous materials, on the contrary these polymer systems even show barrier behavior towards the molecular passage of fluids. However, we have shown the ability towards delamination of layered silicates previously expanded by intercalation of cationic surfactants, after reaction with alkoxides through a sol–gel process [18]. This synthetic route procures the formation of what was firstly called inorganic–inorganic nanocomposites [18] because they showed a whole inorganic nature, but lately has been also referred as delaminated porous clays heterostructures (DPCHs) [31] for its relation with the so-called porous clay heterostructures (PCHs) [32] or just heterostructures or nanorachitectures based on clays [33]. One of their main characteristic is that they often show large specific surface areas and porosity after thermal treatment for removal of the organic matter and consolidation of the inorganic framework generated from the polycondensated alkoxides.
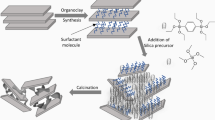
As above indicated the key point to obtain the irreversible delamination of these layered clays is to produce the controlled hydrolysis of alkoxysilanes following spontaneous heterocoagulation processes. Figure 2 schematically shows the overall process which involves two steps. In the first step the organoclay, i.e., alkylammonium-exchanged montmorillonite or vermiculite (e.g., exchanged with cetyltrimethylammonium ions, CTA+), is swollen in an alcohol, e.g., n-butanol, and then is incorporated the alkoxide, e.g., tetramethoxysilane (TMOS) or tetraethoxysilane (TEOS), leaving a certain time to procure the diffusion of the alkoxide to the organophilic part of the organoclay. In a second step is slowly added water to produce the hydrolysis/condensation of the alkoxide which is clearly visualized by a sudden transition from a suspension to a very viscous gel where the magnetic stirrer bar became blocked. A convenient thermal treatment drives to the subsequent elimination of the alkyl-ammonium chains by pyrolysis/combustion and the consolidation of the silica framework, leading to the formation of silica–clay nanocomposites where the polymer is the generated polysiloxane network. The XRD patterns reveal that the delamination degree of smectites depends on the nature of both the starting silicate and the reagent used to generate the silica matrix [19]. In some cases the clay can be completely delaminated even when small clay/silica ratio was used. Specially remarkable is the delamination of vermiculite. This phyllosilicate shows relatively large crystals, the intercalation of a silica network into this silicate producing the permanent separation of lamellae of about 1 nm thick by 50 µm length, i.e., giving materials with an anisotropy ratio as high as 1:50,000. In these SiO2-vermiculite nanoarchitectures is possible to found an increase of the specific surface area from 23 m2/g in the pristine vermiculite to 484 m2/g in the material prepared from TMOS with a 1:1 organoclay/TMOS ratio once heated at 500 °C, which also present a total volume pore of 0.43 cm3/g [19]. The nanoarchitectures based on montmorillonite also show high specific surface areas and porosity that may reach 550 m2/g with pore volume of 0.28 cm3/g in the 0–2 nm pore diameter [19]. Textural changes in the nanocomposites can be also clearly observed from SEM images of the starting silicates compared to those of the nanocomposite precursors, the platelet particles showing a more globular texture with the aspect of a spongy material in which is possible to distinguish small particles of the generated silica. Moreover, TEM characterization supports delamination and so, for instance, the cross-section image of these silica–clay nanocomposites completely resembles to typical images of polymer–clay nanocomposites (Fig. 3) [19].
Scheme of the delamination of alkylammonium-exchanged layered clays (on the left) with alkoxides following a sol–gel process giving rise to intermediate organo-clay materials that after thermal treatment (> 450 °C) in the presence of oxygen leads in a second step to delaminated clay-nanoparticles (NPs) materials. From Ref. [33] - Reproduced by permission of The Royal Society of Chemistry
TEM images of two layered clay-based nanoarchitectures: a a cross-section of a silica–montmorillonite nanocomposite, and b an in-plane detail of a silica–vermiculite nanocomposite (Reprinted from Ref. [19]. Copyright © 2006 WILEY–VCH Verlag GmbH & Co. KGaA, Weinheim)
These silica–clay nanoarchitectures may show hybrid properties from their both components, i.e., the layered silicate and the silica network, exhibiting for instance the cation exchange behavior inherent to the clay substrate [18, 19]. In this way also, the presence of the silica network affords silanol groups offering the possibility to incorporate functionalities by further grafting reactions with diverse organosilanes. Thus, for instance, the treatment of a SiO2-montmorillonite nanoarchitecture with aminopropytrimethoxysilane (APTS) allows the incorporation of amino groups that due to the use of small amounts of HCl in the reaction medium results in the presence of –NH3 +Cl– species that may act as active anion-exchanger sites [19]. Therefore, these functionalized derivatives show retention of both cationic and anionic species, with relatively high ion-exchange capacities (e.g., 55 and 60 meq per 100 g for cations and anions, respectively) as reported by Letaïef and Ruiz-Hitzky [18].
It is worthy to mention that the silica–clay heterostructures in which the alkylammonium ions were not removed by heating may offer other advantages for the use of these types of nanorachitectures. In this way, the presence of the organocations confers hydrophobicity to the interlayer region of the clay, even after delamination by hydrolysis-polymerization of the alkoxysilane takes place. This property has been recently used to incorporate a metallocene catalyst in view to use the resulting material as simultaneous heterogeneous catalyst and nanofiller in the production of polyethylene/clay nanocomposites [22]. The presence of the catalyst incorporated in the silica–clay organoheterostructure allows the polymerization of ethylene within the layers of the clay promoting the dispersion of clay layers in the growing polymer matrix. The molecular weight of the polymers obtained in this way increased ca. 40 % compared to neat polyethylene [22].
Following an analogous procedure to the synthesis of silica-based nanoarchitectures it is possible to prepare titania/ and silica–titania/clay porous nanoarchitectures from montmorillonites and vermiculites exchanged for instance with cetyltrimethylammonium ions, using isopropanol as swelling solvent and titanium tetra-isopropoxide (TTIP) alone or mixed with TMOS mixtures as precursors of the titania or titania–silica networks, respectively [20]. When TTIP is used as the only precursor it is possible to reach the formation of TiO2 nanoparticles (NPs) intercalated into the layered silicates, being possible to reach the stabilization of the anatase phase by a thermal treatment at 500 °C without formation of rutile. In these materials the NPs of crystalline TiO2 present 9–14 nm diameter sizes, depending of the involved clay, and are very homogeneously dispersed as indicated by the TEM images. The nanoarchitectures formed from mixtures of TTIP and TMOS contain silica–titania mixed oxides, in agreement with the absence of peaks in the X-ray diffraction patterns assignable to anatase or to other TiO2 crystalline phase [20]. Here again, it is remarkable the increase of the specific surface area found in the resulting nanoarchitectures compared to the corresponding pristine layered silicates. In particular, the vermiculite derivatives show a BET surface area of 66 m2/g for the titania/clay and 290 m2/g for the silica–titania/clay nanoarchitectures whereas the starting vermiculite shows a value of 23 m2/g. A parallel increase in the porosity is also observed [20]. Photocatalytic properties of the resulting nanoarchitectures have been evaluated following the photodegradation of 2,4-dichlorophenol, showing that these materials can be used for the elimination of organic pollutants. Interestingly the photocatalytic efficiency per mass of TiO2 of these nanoarchitectures seems to be superior to that of commercial P25 from Degussa with the additional advantage that these new photocatalysts can be more easily recovered from the reaction media after reaction [20]. The increase of activity has been attributed to the smaller particle size that disfavor recombination of the photo-excited electrons and holes.
The application of the abovementioned colloidal route to the generation of alumina and silica/alumina NPs must be carefully controlled because of the high reactivity of common Al alkoxides which may hydrolyze before incorporation within the organophilic region of the organoclay [34]. Preparation of porous alumina-montmorillonite heterostructures has been intended using the commercial organoclay known as Cloisite®30B (Southern Clay Products, Rockwood Company, Texas) swollen in n-butanol in the presence of aluminum tri-sec-butoxide (ATSB) alone or mixed with TMOS, being observed that the relative time to reach the sol–gel transition is strongly dependent on the molar ratio of alkoxides in the mixture [21]. Delamination is easily reached for low contents of aluminium however higher contents caused a fast Al3+-alkoxide hydrolysis preventing exfoliation after the calcinations step. In the system containing only aluminum the resulting materials present the clay particles completely collapsed (interlayer basal space of around 1 nm). In general, the presence of silicon alkoxides allows the stabilization of silicoalumina NPs and the formation of porous-clay heterostructures of elevated surface area (200–600 m2/g) and materials with micro- and meso-porosity. The 27Al NMR spectra of these nanoarchitectures indicated that aluminum is in tetrahedral coordination with an environment of SiO2 units, i.e., s corroborating that the use of the silica precursor drives to the formation of silico–alumina [21]. FE-SEM images (Fig. 4) revealed the presence of dispersed silicate layers surrounded by silicoalumina NPs forming phases of spongy-like aspect dispersed within the clay particles, effect that is more evident in nanoarchitectures in which the mesophase formed after the sol–gel transition is freeze-dried instead of dried at 50 °C in air before the calcination step [34]. Another difference between these two types of silica–alumina/montmorillonite nanoarchitectures is that materials derived from mesophases obtained by lyophilization have higher specific surface area and a higher concentration of mesopores with low content in microporosity [34]. The presence of Al in these clay-based nanoarchitectures provide to the resulting materials with acid properties. This property can be modulate varying the Al/Si content and the drying treatment before the calcination step, which is of special relevance in view to their application as catalysts. In this sense, it has been observed that heterostructures prepared from the lyophilized material the content in Brönsted acid centers is substantially higher [34].
As above mentioned the presence of acid sites results of interest for catalytic purposes as for instance in the conversion of limonene to p-cymene and the glycerol recovery reaction [35, 36]. In this way, the catalytic activity of silica–alumina/montmorillonite nanoarchitectures in the conversion reaction of limonene to p-cymene activated by microwave irradiation main reach conversion values higher than 90 % after 15 min of reaction. This behavior is analogous to that described for a commercial silica/alumina (Siral 40) although it can be enhanced at low reaction times being possible to reach 80 % of conversion in 5 min instead of 45 % described by Siral 40. Moreover, these heterostructures also describe best selectivity to p-cymene, getting more than 60 % of p-cymene and lower percentages of by-products [21]. These silica–alumina/montmorillonite nanoarchitectures have been also tested as catalyst in the synthesis of solketal, by acetylation of glycerol. This reaction presents a great relevance because of its use as solvent, as additive for biodiesel fuel and for the production of monoglycerides and other surfactants. This reaction needs very strong acid catalysts to be performed, so to increase the acid sites of the nanoarchitectures it has been required further acid treatment to reach stronger acidity [36]. The activity of the prepared materials compared to that of various homogeneous catalysts (HCl or H2SO4 at 23 %) is lower but it is much higher than commercial acid catalysts (e.g., Amberlist-36), appearing therefore as promising heterogeneous catalysts for this reaction due to the reduction of the corrosion problems involving the use of the homogenous systems.
3 Functionalization of fibrous clays by sol–gel processes
Fibrous clays, sepiolite and palygorskyte, do not offer the possibility of producing delaminated materials since its structure does not consist in a superposition of layers as in the case of the abovementioned phyllosilicates, i.e., smectites and vermiculites. As already indicated, sepiolite and palygorskite exhibit free Si–OH silanol groups, located along the edges of the channels at the external surface (Fig. 1b), that can interact with different types of ion, molecular or polymeric species [37–39]. On the one side, it is well known since many years ago that organosilane coupling agents (typically R–Si–OR or R–Si–Cl) may react with those silanol groups giving rise to stable siloxane bridges [30, 40] .This methodology is commonly applied to render organophilic the highly hydrophilic surface of this silicate, for instance for facilitating further assembling to polymers in the preparation of nanocomposites, and also for incorporating specific functionalities, such as amino, sulphonic, thiol and other organic groups, that can be of interest for developing supported acid catalysts, specific adsorbents or selective sensor devices [41–43]. On the other one side, sepiolite and palygorskite exhibit certain cation-exchange capacity (e.g., in the used sepiolite sample is around 15 mEq/100 g) that is commonly profited for the preparation of organoclays by treatment of the clay with different type of surfactants, the resulting organophilic clays being largely employed in different industrial applications [44–46]. These two characteristics have been of crucial importance in the development of diverse clay-NPs nanoarchitectures by applying a similar colloidal route than adopted in the preparation of the porous nanoarchitectures based on layered clays.
The first assays were applied to the generation of titania NPs on the sepiolite surface [23] in view to its large accessible external surface area (ca. 150 m2/g), which resulted very convenient for the immobilization of those NPs with photocatalytical properties. In this methodology (see in Fig. 5 a scheme of the whole process) sepiolite was firstly treated with cetyltrimethylammonium bromide (CTAB) in view to form the corresponding organoclay in which the CTA+ ions exposed to the exterior of the fiber their long alkyl-chains, creating a highly organophilic interface. The CTA-sepiolite derivative was suspended in iso-propanol and then TTIP or TTIP/TMOS mixtures were added and left to diffuse to the organophilic surface of the clay particles. The addition of small amounts of water provokes the hydrolysis of the alkoxide precursors giving rise to highly viscous colloidal systems till a heterocoagulation process takes place. The recovered solids were dried and thermally treated, driving to the formation of TiO2 NPs of relatively monodiperse particle size that remain homogeneously distributed on the surface of the sepiolite microfibers. The initially amorphous titania phase was transformed in anatase after heating at 500 °C for several hours in air, being observed that the doping with S atoms using thiourea results in an increase in the stability of the anatase phase and a decrease in the excitation band gap energy. The TiO2/sepiolite nanoarchitectures were tested in photocatalytic experiments using phenol as model of pollutant molecule being observed that they are more efficient as photocatalyst systems than the pristine clay and the anatase nanoparticles acting separately, which proved that these materials can be used as improving adsorbents/photocatalysts for environmental applications.
Schematic representation of the sol–gel procedure leading to the formation of TiO2/sepiolite nanoarchitectures by the reaction of TTIP with controlled amounts of water at the CTA-sepiolite interface and further calcinations to promote the TiO2 NPs formation. Reprinted with permission from Ref. [23]. Copyright (2008) American Chemical Society
In the same way it is possible to promote the formation of alumina and silica–alumina NPs on the surface of sepiolite, leading to the development of materials of interest as acid catalysts [27]. In the present case it have been used a commercial organosepiolite known as Pangel B-40, which is commercialized by TOLSA SA (Spain), and 2-butanol as solvent. In contrast to the preparation of nanoarchitectures from montmorillonite, in the present case it is possible to produce the corresponding heterostructures just containing Al2O3 NPs, probably due to the easier access of the aluminium alkoxide to the organophilic interphase of the organosepiolite which impedes its hydrolysis. Besides, it can be considered the role of sepiolite as a strongly dehydrating material producing the scavenging of water molecules from the reaction media preventing the hydrolysis of the precursor. In spite of this the sol–gel transition is very rapid when is only used aluminium tri-sec-butoxide, becoming slower the process when TMOS is incorporated in the reaction. FTIR and solid-state 29Si NMR spectroscopies allow to interpret the formation of alumina or silica–alumina NPs that after heating of the samples at 550 °C condensate with the silanol groups at the external surface of sepiolite remaining bonded to the clay fibers through Si–O–Al bridges. 27Al NMR spectroscopy reveals the presence of [AlO4] units bonded to Si atoms as occurs in amorphous aluminosilicates and silica–alumina gels [47, 48], confirming that the use of mixtures of Al and Si alkoxide precursors drive to the formation of true silica–alumina mixed oxides. In samples with high content in Al it is even observed the presence of pentacoordinated aluminium which has been attributed to formation of Si–O–Al bonds between the silanol groups of sepiolite and the alumina upon the thermal treatment [27]. One of the most interesting characteristics of these materials is their large surface area and porosity, which can be tuned by varying the content in Si. Thus, high silica contents generated microporous systems, whereas the incorporation of alumina enhances the mesoporous formation. The possibility of varying the Si–Al content on the NPs linked to the surface of sepiolite also permits the tuning of the surface acidity, being determined values that increase from 0.1 to 1.4 mmol of acid sites per gram with the aluminium content [27]. This acid properties of the developed Al2O3/ and SiO2–Al2O3/sepiolite nanoarchitectures result very attractive in view to application of these materials in acid catalysis. In this regard, acid activity in the 2-propanol dehydration test reveals high selectivity of these catalysts towards propylene, reaching conversion values close to 60 % in the case of the heterostructures with the highest aluminum content [27].
As abovementioned, the role of the organophilic interface of the clay plays a key role in the development of this type of nanorachitecture, which differences this methodology from other approaches reported in the literature in which clays are directly mixed or assembled to alkoxide precursors or its gels at their earliest stage of hydrolysis-polycondensation process. The effect of the interface provided by the organocations assembled to the clay surface has been recently reported in a study in which it has been analyzed the role of both the nature of the organosepiolite and the silica precursor in the characteristics of the final nanoarchitectures [26]. In this case it was used organosepiolites containing propylamonium, cetyltrimethylammonium, didodecyldimethyl ammonium and methylbenzylbis (hydrogenated tallow alkyl) ammonium ions (this last one is the abovementioned Pangel B-40 commercial clay from TOLSA SA) using TMOS, TEOS, tetrabutyl ortosilicate (TBOS) and sodium silicate as the silica precursors. In this case it was used isopropanol as solvent and ultrasound irradiation to favor a rapid homogenization of the systems and the activation of the sol–gel transition. The relative ratio of organoclay to silica source was also varied in view to determine the nature of the formed nanoarchitectures. The first observation was that sodium silicate was not an adequate precursor as it rapidly precipitates resulting in the formation of heterogeneous materials. The combination of TMOS and the organosepiolite containing CTA+ ions in different ratio (chosen to reach SiO2:sepiolite ratio of 1:1, 2:1, 3:1 and 6:1, assuming a complete hydrolysis-polycondensation of the silica precursor) results in clearly different nanoarchitectures as revealed FE-SEM and TEM images (Fig. 6). In this sense, the nanoarchitecture prepared with the lowest amount of silica precursor shows sepiolite fibers with an irregular coverage in which it is difficult to distinguish the NPs (1:1 ratio), which are more clearly distinguished as the content of silica precursor increases (2:1) being possible to find them cemented (3:1). When the amount of TMOS is very large (6:1 ratio) a part of alkoxide precursor probably remain outside the organophilic region generating silica NPs not assembled directly to sepiolite but giving rise to globular forms of silica NPs sometimes bonding and agglomerating sepiolite particles [26]. The agglomeration of sepiolite fibers is also observed when the silica precursor is very big, as it is the case of TBOS, which is not completely accommodate in the organophilic interface of the organosepiolite giving rise to the agglomeration of sepiolite fibers covered by thick and homogeneous coatings of silica NPs.
FE-SEM (left) and TEM (right) images of four SiO2/sepiolite nanoarchitectures prepared from a organosepiolite containing CTA+ ions and TMOS in different ratio a 1:1, b 2:1, c 3:1 and d 6:1. Reprinted with permission from Ref. [26]. Copyright American Scientific Publishers
Interestingly, the study of the influence of the nature of the organocation carried out with a system in which it is employed TMOS as precursor to generate nanoarchitectures with a 2:1 silica:sepiolite ratio, reveals the existence of a templating effect. Thus, the organosepiolite containing propylammonium ions, i.e., the smallest organocation, evidences difficulties to procure the necessary organophilic environment for the incorporation of TMOS and the coverage of the fibers is very irregular. Organo-sepiolites contain long-chain branched organocations, e.g., didodecyldimethyl ammonium and methylbenzylbis(hydrogenated tallow alkyl)ammonium ions, allow the accommodation of larger amounts of TMOS and therefore the fibers clearly show thicker coatings of silica NPs than in the case of the nanoarchitecture based on sepiolite containing CTA+ ions [26]. It is worthy to mentioned that these nanoarchitectures also show significant differences in their textural properties and so, for instance, the nanoarchitectures based on sepiolite containing branched organocations show high values of the total pore volume which has been associated with the formation of a “house of cards” arrangement that favors the creation of macropores as occurs in other clay-based materials [49, 50]. In contrast the nanoarchitecture based on sepiolite containing CTA+ ions show a relatively high surface area 340 m2/g without appreciable microporosity, being the developed surface area only related to the presence of mesoporosity [26]. This last observation may be related to a possible role of the surfactant which may direct the organization of the silica precursor which after the removal of the CTA+ ions originates this mesoporosity as it does in the formation of MCM-41 materials and other mesoporous solids [51]. This observation could be of special relevance in view to adopt the here reported methodology in the preparation of new nanoarchitectures with assembled template NPs.
Sepiolite and palygorskite are becoming very interesting fibrous nanofillers for the preparation of diverse polymer–clay nanocomposites [38, 39]. In view of that SiO2/sepiolite nanoarchitectures before and after heating treatment for elimination of the organocations have been tested as nanofiller in epoxy resins. In this study it has been observed that both types of nanoarchitectures, i.e., with and without organocations, show a mechanic reinforcement effect when incorporated in the resin matrix, although the presence of the organic moieties in the intermediate organo-heterostructures promotes a more effective adhesion between the dispersed and the continuous phases [26]. This result suggests that the presence of the surfactant may act as interfacial agent which may be useful for its compatibilization with polymeric matrices. Considering this result and the huge chemistry related to silica it could be envisaged a great potential of these novel nanoarchitectures in the development of improving functional materials for applications in many diverse fields.
4 Conclusions and perspectives
The here reported sol–gel route for the preparation of porous clay derivatives offers significant differences with respect to common ways previously applied for the synthesis of PILCs and PCHs as well as to other approaches based on the direct combination of metal alkoxides and clays. In this way, the use of organoclays offers advantages because the organocations act as an organophilic interphase in which alkoxysilanes and/or metal alkoxides can accommodate giving rise the hydrolysis/policondensation processes in a controlled manner. In this way, these systems result in nanoarchitectures consisting in NPs assembled to the layers or fibres of the involved clay, which at the same time becomes delaminated. Delamination of layered silicates in the sol–gel process involving alkoxides offers the possibility to develop silica-based porous heterostuctures (inorganic–inorganic nanocomposites) provided of functions that could be introduced directly or in a post-synthesis step. The present results open a way to develop other new porous nanoarchitectures, using as precursors different metal alkoxydes (e.g., Ti, Al, Zr, Sn, Ge,…) as well as diverse type of layered solids (alkaline silicates, titanates, phosphates, chalcogenides,…). On the other hand, fibrous clays, specially sepiolite, offers a good opportunity for the assembly of particles of different nature due to the presence of silanol groups in their external surface. In this way, many opportunities related to both the preparation of new nanoarchitectures with functional properties and the exploration of applications by using principles of the proposed route, for instance for optic and magnetic applications by assembling quantum dots or iron oxide NPs. In fact, recently other authors have applied this same route to the preparation of diverse nanoarchitectures based on the use of beidellite instead of montmorillonite [52] or palygorskite instead of sepiolite for incorporation of TiO2 NPs [53] or to explore the incorporation of other type of NPs as for instance Au by previous modification of sepiolite by grafting of an organosilane that act as bonding sites for the growing of the NPs [54]. Anyway, it is expected that, the nature of the lamellar or fibrous precursor, as well as the associated materials, is decisive with respect to the properties and therefore their application in practical uses. Subsequently, the impact and prospect of application of these materials is large with possible incidence in areas as diverse as adsorption (e.g., gas separation and purification at industrial scale, catalysis (e.g., photocatalysis, wet hydrogen peroxide catalytic oxidation, etc.), nanofillers of polymers, membranes, photoactive materials, for instance by incorporation of quantum dots, etc., where the characteristics of involved clays, non-polluting and cost reduced may be an additional advantage.
References
Bruce DW, O’Hare D, Walton RI (eds) (2010) Porous materials. Vol 5 of inorganic materials series. Wiley, Chichester
Su BL, Sanchez C, Yang XY (eds) (2011) Hierarchically structured porous materials: from nanoscience to catalysis, separation, optics, energy, and life science. Wiley, Weinheim
Medina ME, Platero-Prats AE, Snejko N, Rojas A, Monge A, Gándara F, Gutiérrez-Puebla E, Camblor MA (2011) Adv Mater 23:5283–5292
Thomas JM, Thomas WJ (1997) Principles and practice of heterogeneous catalysis. VCH, Weinheim
Díaz U, Brunela D, Corma A (2013) Chem Soc Rev 42:4083–4097
Occelli ML, Kessler H (eds) (1997) Synthesis of porous materials — zeolites, clays, and nanostructures. Marcel Dekker, New York
Bergaya F, Lagaly G (eds) (2013) Handbook of clay science, 2nd edn. Elsevier, Oxford
Plee D, Borg F, Gatineau L, Fripiat JJ (1985) J Am Chem Soc 107:2362–2369
Mitchell IV (ed) (1990) Pillared layered structures. Current trends and applications. Elsevier, London
Gil A, Korili SA, Trujillano R, Vicente MA (eds) (201) Pillared clays and related catalysts. Springer, New York
Vicente MA, Gil A, Bergaya F (2013) In: Bergaya F, Lagaly G (eds) Handbook of clay science, 2nd edn. Elsevier, Oxford, pp 523–558
Lagaly G (1986) Solid State Ionics 22:43–51
Ruiz-Hitzky E, Aranda P, Serratosa JM (2004) In: Auerbach SM, Carrado KA, Dutta PK (eds) Handbook of layered materials. Marcel Dekker, New York, pp 91–154
Lagaly G, Ogawa M, Dékány I (2013) In: Bergaya F, Lagaly G (eds) Handbook of clay science, 2nd edn. Elsevier, Oxford, pp 435–506
Ruiz-Hitzky E, Aranda P, Darder M, Rytwo G (2010) J Mater Chem 20:9306–9321
Sayari A, Jaroniec M (eds) (2008) Nanoporous materials. World Scientific, Singapore
Zebib B, Zeng S, Krafft JM, Lambert JF, Blanchard J, Nie H, Li D, Breysse M (2005) Stud Surf Sci Catal 158:517–524
Letaïef S, Ruiz-Hitzky E (2003) Chem Commun: 2996–2997
Letaïef S, Martín-Luengo MA, Aranda P, Ruiz-Hitzky E (2006) Adv Funct Mater 16:401–409
Manova E, Aranda P, Martín-Luengo MA, Letaief S, Ruiz-Hitzky E (2010) Micro Meso Mater 131:252–260
Belver C, Aranda P, Martín-Luengo MA, Ruiz-Hitzky E (2012) Micro Meso Mater 147:157–166
Zapata P, Belver C, Quijada R, Aranda P, Ruiz-Hitzky E (2013) Appl Cat A Gen 453:142–150
Aranda P, Kun R, Martin-Luengo MA, Letaïef S, Dékány I, Ruiz-Hitzky E (2008) Chem Mater 20:84–89
González-Alfaro Y, Aranda P, Fernandes FM, Wicklein B, Darder M, Ruiz-Hitzky E (2011) Adv Mater 23:5224–5228
Ruiz-Hitzky E, Darder M, Fernandes FM, Zatile E, Palomares FJ, Aranda P (2011) Adv Mater 23:5250–5255
Gómez-Avilés A, Aranda P, Fernandes FM, Belver C, Ruiz-Hitzky E (2013) J Nanosci Nanotechnol 13:2897–2907
Belver C, Aranda P, Ruiz-Hitzky E (2013) J Mater Chem A 1:7477–7487
Van Olphen H (1977) An introduction to clay colloid chemistry, 2nd edn. Wiley, New York
Alexandre M, Dubois P (2000) Mater Sci Eng 28:1–63
Ruiz-Hitzky E, Van Meerbeeck A (2006) In: Bergaya F, Theng BKG, Lagaly G (eds) Handbook of clay science. Elsevier, Amsterdam, pp 583–621
Aranda P, Belver C, Ruiz-Hitzky E (2013) In Aranda P, Ogawa M, Drummy LF (eds) Clays and materials, CMS Workshop lectures series Vol. 18. The Clay Minerals Society, Chantilly, VA, USA, in press
Galarneau A, Barodawalla A, Pinnavaia TJ (1995) Nature 374:529–531
Ruiz-Hitzky E, Aranda P, Belver C (2012) In: Ariga K (ed) RSC Nanoscience & nanotechnology 24: manipulation of nanoscale materials: an introduction to nanoarchitectonics. Royal Society of Chemistry, Cambridge, pp 87–111
Belver C, Aranda P, Martín-Luengo MA, Ruiz-Hitzky E (2010) In: Actes du Congres Materiaux 2010. Fédération Française pour les Sciences de la Chimie, Nantes 2010: e-proceedings 1635-Aranda-Pilar, pp 1–4
Belver C, Molinero L, Ladero M, Aranda P, Ruiz-Hitzky E (2010) In: Book of Abstracts of the 2010 SEA-CSSJ-CMS Trilateral Meeting on Clays General Meeting, Seville, Spain, pp 345–346 http://www.sea-arcillas.es/publicaciones/2010%20SEA-CSSJ-CMS%20Trilateral%20Meeting%20on%20Clays.pdf. Accessed 25 Sept 2013
Belver C, Esteban J, Ladero M, Aranda P, Ruiz-Hitzky E (2011) In: Book of abstracts of the Europacat X, European on Catalysis Congress, Glasgow, United Kingdom, PTh121
Ruiz-Hitzky E (2001) J Mater Chem 11:86–91
Ruiz-Hitzky E, Aranda P, Alvarez A, Santarén J, Esteban-Cubillo A (2011) In: Galán E, Singer A (eds) Developments inpalygorskite-sepiolite research. A new outlook on these nanomaterials. Elsevier, Oxford, pp 393–452
Ruiz-Hitzky E, Aranda P, Darder M, Fernandes FM (2013) In: Bergaya F, Lagaly G (eds) Handbook of clay science, 2nd edn. Elsevier, Oxford, pp 721–743
Ruiz-Hitzky E, Fripiat JJ (1976) Clays Clay Miner 24:25–30
Aznar AJ, Ruiz-Hitzky E (1988) Mol Cryst Liq Cryst Inc Nonlinear Opt 161:459–469
Celis R, Hermosín MC, Cornejo J (2000) Environ Sci Technol 34:4593–4599
Gómez-Avilés A, Darder M, Aranda P, Ruiz-Hitzky E (2007) Angew Chem Int Ed 46:923–925
Alvarez A, Santaren J, Perez Castell R, Casal B, Ruiz-Hitzky E, Levitz P, Fripiat JJ (1977) In Schultz LG, van Olphen H, Mumpton FA (eds) Proceedings International Clay Conference, Denver 1985. Clay Minerals Societ,: Bloomington IN, USA, pp 370–374
Alvarez A, Santaren J, Esteban-Cubillo A, Aparicio P (2011) In: Galán E, Singer A (eds) Developments inpalygorskite-sepiolite research. A new outlook on these nanomaterials. Elsevier, Oxford, pp 281–298
Shuali U, Nir S, Rytwo G (2011) In: Galán E, Singer A (eds) Developments inpalygorskite-sepiolite research. A new outlook on these nanomaterials. Elsevier, Oxford, pp 351–374
Bravo-Zhivotovskii D, Melamed S, Apeloig Y, Schmidt A, Melchior S, Shter GE, Grader GS (2001) Chem Mater 13:247–249
Lartiges BS, Bottero JY, Derrendinger LS, Humbert B, Tekely P, Suty H (1997) Langmuir 13:147–152
Pinnavaia TJ, Tzou MS, Landau SD, Raythatha H (1984) J Mol Catal 27:195–212
Luckham PF, Rossi S (1999) Adv Colloid Interf Sci 82:43–92
Kresge CT, Leonowicz ME, Roth WJ, Vartuli JC, Beck JS (1992) Nature 359:710–712
Bouna L, Rhouta B, Amjoud M, Maury F, Jada A, Daoudi L, Senocq F, Lafont M-C, Drouet C (2012) Matér Techniq 100:241–252
Bouna L, Rhouta B, Amjoud M, Maury F, Lafont M-C, Jada A, Senocq F, Daoudi L (2011) Appl Clay Sci 52:301–311
Letaief S, Grant S, Detellier C (2011) Appl Clay Sci 53:236–243
Acknowledgments
Financial support from the Comision Interministerial de Ciencia y Tecnología (Spain; MAT2012-31759 project) is acknowledged. Authors also thank to the European Union COST Action MP1202.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ruiz-Hitzky, E., Aranda, P. Novel architectures in porous materials based on clays. J Sol-Gel Sci Technol 70, 307–316 (2014). https://doi.org/10.1007/s10971-013-3237-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-013-3237-9