Abstract
Barium titanate ceramics were prepared using the nanopowder resulting from a polymeric precursor method, a type of modified Pechini process. The obtained nanopowder was observed to agglomerate and in order to de-agglomerate the powder and enhance the properties of the barium titanate the material was attrition milled. The impact of this attrition milling on the electrical properties of the barium titanate was analysed. The temperature dependence of the relative dielectric permittivity showed three structural phase transitions that are characteristic for ferroelectric barium titanate ceramics. The relative dielectric permittivity at the Curie temperature was higher for the attrition-treated sample than for the non-treated barium titanate. The dielectric losses were below 0.04 in both barium titanate ceramics. The grain and grain-boundary contributions to the total resistivity were observed using impedance analyses for both ceramics. A well-defined ferroelectric hysteresis loop and piezoelectric coefficient d33 = 150 pC/N were obtained for the ceramics prepared from the de-agglomerated powder. In this way we were able to demonstrate that by attrition milling of chemically obtained powders the ferroelectric and piezoelectric properties of the ceramics could be enhanced.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Barium titanate has a special place in the group of ferroelectrics because it can be formulated in a large number of systems and solid solutions that provide a wide range of applications. Over the years barium titanate has been used for the fabrication of ceramic capacitors, positive temperature coefficient of resistivity thermistors, piezoelectric sensors, optoelectronic devices, transducers, actuators, etc. [1, 2].
There is currently a demand for the fabrication of powders <100 nm to allow the production of thinner layers for multi-layer capacitors and cheaper or more reliable routes than those that are presently available. In order to prepare nanopowders with good characteristics, different chemical methods have been studied. The chemical synthesis of barium titanate has developed through techniques such as sol–gel, coprecipitation, and hydrothermal and polymeric precursor methods [1]. The advantage of chemical methods is the quasi-atomic dispersion of the constituent components in the liquid precursor, which facilitates the synthesis of crystallized powder with submicron particles and high purity at low temperatures. The main problem with chemical routes is the agglomeration of the resulting fine particle powders, which can affect the ceramics’ preparation as well as the properties.
Control of the microstructure in ceramics is very important because it has a major effect on the properties. In order to obtain ceramic materials with the desired properties each step in the processing has to be monitored [3, 4]. The most common factor that can influence the final properties of a material is the agglomeration of powders, influenced by the powder synthesis method, especially when nanopowders are prepared. Therefore, an additional milling step after the synthesis could be added to avoid the negative effects of agglomeration on the properties of the materials.
In this study, barium titanate nanopowders were de-agglomerated by attrition milling and the positive effect of this process on the dielectric, ferroelectric and piezoelectric properties of the obtained ceramics was analyzed.
2 Experimental procedure
Nanopowders of pure barium titanate BT were prepared by a modified Pechini method. Titanium tetra-isopropoxide (Ti[OCH(CH3)2]4, Alfa Aesar, 99.995 %) and barium acetate (Ba(CH3COO)2), Alfa Aesar, 99.0–102.0 %) were used as sources of the metal ions. Solutions of titanium citrate and barium citrate were prepared using ethylene glycol (EG) and citrate acid (CA) as solvents (M (metal ion):CA:EG = 1:4:16). These solutions were mixed and heated until the mixture changed to a glassy resin. Subsequent processing is based mainly on the decomposition of the organic material during the whole range of calcinations steps from 250 to 800 °C [5, 6].
Since the nanopowders obtained by chemical methods are usually highly agglomerated, such a powder was treated in an attrition mill (PE075 Netzsch, Germany) with zirconia media for 1 h in 2 % polyacrylic acid. The milled barium titanate powder (BTA) was then dried at 150 °C and used for subsequent investigations. In order to see the influence of the attrition milling on the barium titanate’s microstructure and electrical properties a complete analysis of the BT and BTA ceramics was conducted.
The nanopowders of BT and BTA were uniaxially pressed at 196 MPa into discs of 10 mm in diameter. Sintering was performed at 1,300 °C for 8 h with a heating rate of 10 °C/min. Scanning electron microscopy (SEM Tescan VEGA TS 5130MM) was used to analyze the microstructure. The median grain size was determined from SEM images containing more than 100 grains and expressed as Feret’s diameter. The density of the barium titanate ceramics was obtained geometrically. The samples were prepared for electrical measurements by polishing, followed by applying silver electrodes on both sides of the samples. Measurements of the dielectric properties versus temperature were carried using a LCR meter (model 4284 A, Hewlett-Packard) at a frequency of 1 kHz. The dielectric loss tangent of the barium titanate ceramics was derived by calculating tan δ = ε″/ε′.
The impedance measurements were also carried out at 300 °C in the frequency range 42 Hz–1 MHz using a HIOKI 3532-50 LCR HiTester. To obtain continuous metallic contacts, Pt paste was deposited on the polished surfaces of the BT and BTA ceramics. All the collected data were analyzed using the commercial software package Z-view.
The electrical polarization (P) and strain (S) were measured with respect to the electrical field (E) using the commercial setup Aix-PES (Aixacct Systems, Aachen, Germany) using a bipolar 1-Hz sine-wave input. All the measurements were made using an electric field with an amplitude of 30 kV/cm. The samples were poled in silicon oil with a DC electric field of 2 kV/mm at 100 °C for 2 min. After poling the samples were aged for 24 h and the piezoelectric coefficient d33 was measured at a frequency of 50 Hz using a Berlincourt piezometer (Take Control PM10, Birmingham, UK).
3 Results and discussion
Cubic barium titanate nanopowder was prepared by the polymeric precursor method (a modification of the Pechini process) [7]. The Pechini process allows good homogeneity and purity with respect to the desired chemical composition. Barium titanate nanopowder with primary particles of ~74 nm was obtained. The main problem that appears with chemically prepared nanopowders is the high levels of agglomeration caused by very small primary particles that tend to join together. Detailed analyses of BT nanopowders shown in a previous report [7] indicated the agglomeration of the prepared BT powders. The strong influence of agglomeration on the properties of nanopowders and ceramics was noticed by other authors for different materials [4, 8]. In order to diminish the number and the dimensions of the agglomerates, attrition milling was performed. After milling for 1 h the de-agglomeration of the powders was evident and indicated by a decrease in the agglomeration factor by almost 85 %. A decrease in the particle size (from 74 to 50 nm) and an increase in the specific surface area (from ~13 to 20 m2/g) in the attrition-treated powders were noticed [7]. The prepared BT and BTA powders were sintered at 1,300 °C for 8 h and a tetragonal crystal structure was obtained in both ceramic samples [7]. In order to see the impact of the agglomeration of the nanopowders on the electrical properties of the obtained ceramics both types of ceramics were analyzed.
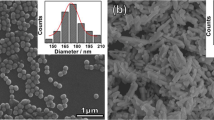
Figure 1 shows SEM micrographs of the BT and BTA ceramics. Polygonal grains and microstructures with different grain size distributions were obtained. The grain size distributions in the BT and BTA ceramics are presented in Fig. 2. The average grain sizes of both ceramics were similar, i.e., 2.96 ± 1.35 and 2.33 ± 0.99 μm for the BT and BTA, respectively. However, a more uniform microstructure can be observed for the BTA than for the BT ceramics and the density of the BTA ceramics was found to be higher (~95 % of theoretical value) than that obtained for the BT ceramics, i.e., ~90 %.
The temperature dependence of the relative dielectric permittivity of barium titanate ceramics at different frequencies is presented in (Fig. 3). The three phase transitions—cubic to tetragonal (TC−T), tetragonal to orthorhombic (TT−O), orthorhombic to rhombohedral (TO−R)—characteristic for barium titanate can be observed for both BT ceramics on the curve typical for classic ferroelectric barium titanate material (Table 1). Both exhibits a sharp permittivity maximum at TC, but it is located at a slightly higher temperature for BTA, at around 123 °C, compared to the 120 °C found for BT. The relative dielectric permittivity value at the Curie temperature increased from 1,340 to 6,700 after attrition milling (Table 1). Furthermore, the possible factors that influence the ε′ enhancement could be a more homogeneous microstructure and a higher density obtained for the BTA ceramics [9]. Figure 3 presents the temperature dependence of tan δ. All three phase transitions can be observed in the diagrams. At temperatures above 150 °C an increase of tan δ can be observed. A certain frequency dispersion in the paraelectric state was noticeable, indicating a thermally activated Maxwell–Wagner relaxation [10, 11]. The values of tan δ were under 0.04 for both barium titanate ceramics across the whole of the measured temperature range.
Impedance spectroscopy(IS) was used to evaluate and separate the contributions of various components, such as the grain, grain boundary and electrode, to the overall electrical properties of the barium titanate ceramics. This analysis is a useful tool for investigating the electrical homogeneity of the ceramics [12, 13]. A major problem in the characterizing of pure barium titanate is that the resistivity is too high to be measured at temperatures below 200 °C [12]. Therefore, complex impedance plane plots of data were collected at 300 °C (Fig. 4). Two well-resolved semicircular arcs are observed for both BT samples. It is believed that the low-frequency semicircle corresponds to the grain-boundary contribution and the high-frequency semicircular arc is attributed to the grain contribution. The absence of a third semicircle suggests that the contribution of the electrode-materials interface to the impedance is negligible in the observed frequency range. Perhaps the measurements at much lower frequencies could enable the detection of this contribution. The equivalent circuit consisted of two parallel RC elements connected in series and Z-view fitting software was used to evaluate the values of Rg and Rgb. It is apparent from the extracted resistivity data that the attrition milling of powders causes an increase in the grain and grain-boundary resistivity of barium titanate ceramics. These results are in agreement with the study of Thakur et al. on barium titanate obtained by a solid-state reaction, also treated in an attrition mill [14]. The impedance spectroscopy analysis showed much higher grain and grain-boundary resistivities, also indicating that Rgb ≫ Rg. Cgb has a higher value in comparison with Cg in both ceramics, which is in accordance with the brick-layer model for electroceramics that shows Cgb ≫ Cg [15]. On the other hand, the BTA ceramic has shown higher values of both types of capacitance compared to the BT ceramic, indicating the formation of thicker grain boundaries that act as a barrier to the cross transport of the charge carriers (Table 2).
The P–E hysteresis loops were measured at room temperature for the BT and BTA ceramics and are shown in (Fig. 5) For comparison, the loops were measured at the same maximum electric field of 30 kV/cm. The ferroelectric behavior of BTA ceramics is clearly observed: the remnant polarization (Pr) and the coercive field (Ec) are 11.5 μC/cm2 and 2.8 kV/cm, respectively. Comparing with the literature data, BTA ceramics show good ferroelectric behavior. The Pr value of the BTA ceramics is higher than the previously reported values, as shown in Table 3. On the other hand, the P–E measurements of the BT ceramics indicate higher conductivity than for the BTA and hence in this case the Pr and Ec cannot be determined (see Fig. 5).
In parallel with the polarization measurements the strain (S) versus electric field (E) was measured at 30 kV/cm, and is shown in Fig. 6. The S–E loops of both ceramics have a “butterfly” shape, resulting from the superimposed piezoelectric and domain-switching effects. The same butterfly loop shape was previously reported for barium titanate single crystals [16] and ceramics [17]. The measured peak-to-peak strains (Spp) of the BTA and BT ceramics are markedly different. The largest Spp, i.e., 0.075 %, was obtained for the BTA ceramics, while for the BT ceramics the Spp is much smaller, i.e., 0.025 %. The asymmetry of the butterfly loop of the BTA ceramics could be related to the partial poling of the sample during a measurement with a maximum field of 30 kV/mm, as previously shown in [16] for single crystals.
The measured piezoelectric coefficient d33 of the poled BTA ceramics was 150 pC/N, which is comparable or even higher than the previously reported value for barium titanate ceramics [16, 18], but still lower than the value reported in [20]. In Ref. [21] it was shown that the piezoelectric constant d33 of barium titanate ceramics can be affected by the ferroelectric domain structure, i.e., with a decrease of the domain size the piezoelectric coefficient d33 is enhanced. On the other hand, we were not able to pole the BT ceramics due to the lower break-down field and the higher conductivity of the pellets (as observed in the P–E measurement). These facts indicate the importance of the de-agglomeration of the powder during the preparation procedure for barium titanate ceramics.
4 Conclusion
Agglomerated cubic barium titanate powders were prepared by a modified Pechini process. Reducing the size and the number of agglomerates was achieved by attrition milling for 1 h. In order to see the impact of the additional milling step on the properties of the resulting ceramics, both types of ceramics prepared from the agglomerated BT and de-agglomerated BTA powders were analyzed. The dielectric permittivity measurements showed a significant improvement in the properties of the barium titanate. The values of the relative dielectric permittivity were 6,700 and 1,340 for the BTA and BT ceramics, respectively. The reason could be the formation of a more homogeneous and dense microstructure in the BTA ceramics. The impedance spectroscopy analysis showed much higher grain and grain-boundary resistivities, also indicating that Rgb ≫ Rg. On the other hand, the obtained capacity appears to be higher in the attrition milled material, showing Cgb ≫ Cg, which is in agreement with the brick-layer model for electroceramics with insulating grain boundaries. The equivalent circuit that consists of two parallel RC elements connected in series was found to be suitable for the investigated barium titanate ceramics.
The well-defined and saturated P–E hysteresis loop of the BTA ceramics confirmed the existence of ferroelectric properties for this material. The remnant polarization Pr and the coercive field Ec of the BTA ceramics were 11.5 μC/cm2 and 2.8 kV/cm, respectively. The value of Pr is much higher than that previously reported in the literature. The piezoelectric coefficient d33 of the BTA ceramics was 150 pC/N. The high ferroelectric and piezoelectric properties of the BTA ceramics in comparison with the BT ceramics indicate the importance of de-agglomeration of the powder during the preparation procedure for barium titanate.
References
Jona F, Shirane G (1993) Ferroelectric crystals. Dover Publications, New York
Moulson AJ, Herbert JM (2003) Electroceramics, 2nd edn. Wiley, England
Shiau FS, Fang TT, Leu TH (1998) Effects of milling and particle size distribution on the sintering behavior and the evolution of the microstructure in sintering powder compacts. Mater Chem Phys 57:33–40
Tusseau-Nenez S, Ganne JP, Maglione M, Morell A, Niepce JC, Pate M (2004) BST ceramics: effect of attrition milling on dielectric properties. J Eur Ceram Soc 24:3003–3011
Pechini MP, Adams N (1967) United States patent No 3, 330, 697
Ramajo L, Parra R, Roboredo M, Zaghete M, Castro M (2008) Heating rate and temperature effect on the BaTiO3 formation by thermal decomposition of (BaTi) organic precursors during the pechini process. Mat Chem Phys 107:110–114
Vijatović Petrović MM, Bobić JD, Radojković AD, Banys J, Stojanović BD (2012) Improvement of barium titanate properties induced by attrition milling. Ceram Inter 38:5347–5354
Dabhade VV, Rama Mohan TR, Ramakrishnan P (2007) Nanocrystalline titanium powders by high energy attrition milling. Powder Technol 171:177–183
Thakur OP, Prakash C, James AR (2009) Enhanced dielectric properties in modified barium titanate ceramics through improved processing. J Alloys Compd 470:548–551
Horchidan N, Ianculescu A, Curecheriu L, Tudorache F, Musteata V, Stoleriu S, Dragan N, Grisan D, Tascu S, Mitoseriu L (2011) Preparation and characterization of barium titanate stannate solid solutions. J Alloys Compd 509:4731–4737
Vijatovic Petrovic MM, Bobic JD, Ramoska T, Banys J, Stojanovic BD (2011) Antimony doping effect on barium titanate structure and electrical properties. Ceram Int 37:2669–2677
West AR, Sinclair DC, Hirose N (1997) Characterization of electrical materials, especially ferroelectrics by impedance spectroscopy. J Electroceram 1(1):65–71
Devi S, Jha AK (2009) Phase transition and electrical characteristics of tungsten substituted barium titanate. Physica B 404:4290–4294
Thakur OP, Feteira A, Kundys B, Sinclair DC (2007) Influence of attrition milling on the electrical properties of undoped-BaTiO3. J Eur Ceram Soc 27:2577–2589
Sinclair DC, West AR (1989) Impedance and modulus spectroscopy of semiconducting BaTiO3 showing positive temperature coefficient of resistance. J Appl Phys 66:3850–3856
Jaffe B, Cook WR, Jr, Jaffe H (1971) Piezoelectric ceramics. Academic press, London
Sonia Patel RK, Kumar P, Prakash C, Agrawal DK (2012) Low temperature synthesis and dielectric, ferroelectric and piezoelectric study of microwave sintered BaTiO3 ceramics. Ceram Int 38:1585–1589
Chandramani Singh K, Nath AK (2011) Barium titanate nanoparticles produced by planetary ball milling and piezoelectric properties of corresponding ceramics. Mater Lett 65:970–973
Thakur OP, Prakash C, Agrawal DK (2002) Structural and electrical properties of microwave-processed BaTiO3 ceramics. J Ceram Process Res 3:75–79
Shao S, Zhang J, Zhang Z, Zheng P, Zhao M, Li J, Wang C (2008) High piezoelectric properties and domain configuration in BaTiO3 ceramics obtained through the solid-state reaction route. J Phys D Appl Phys 41:125408
Takahashi H (2012) Development of Lead-Free BaTiO3 ceramics possessing enhanced piezoelectric properties. Electron Commun Jpn 95([4):1168–1173
Acknowledgments
The authors gratefully acknowledge the Ministry of Education, Science and Technological Development of the Republic of Serbia for the financial support of this work (projects III45021) and the COST MP0904 Action SIMUFER: “Single- and multiphase ferroics and multiferroics with restricted geometries”. Special thanks to Dr Paul Bowen from EPFL, Lausanne, Switzerland for generous research support. Technical support by David Žehelj from JSI is gratefully acknowledged. H. Uršič thanks the Slovenian Research Agency for the financial support in the frame of programs Electronic Ceramics, Nano-, 2D and 3D Structures (P2-0105).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vijatović Petrović, M.M., Bobić, J.D., Uršič, H. et al. The electrical properties of chemically obtained barium titanate improved by attrition milling. J Sol-Gel Sci Technol 67, 267–272 (2013). https://doi.org/10.1007/s10971-013-3075-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-013-3075-9