Abstract
The partial substitution of CaF2 for CaO in the Na2O–CaO–SiO2–P2O5 system was conducted by the sol–gel method and a comparison of the glass–ceramic properties was reported. Based on thermogravimetric and differential thermal analysis, the gels were sintered with a suitable heat treatment procedure. The glass–ceramic properties were characterized by X-ray diffraction, fourier-transform infrared spectroscopy, scanning electron microscopy, energy dispersive spectrometer and so on, and the bioactivity of the glass–ceramic was evaluated by in vitro assays in simulated body fluid. Results indicate that with the partial substitution of CaF2 for CaO in glass composition, the volume density, apparent porosity, bending strength and microhardness of the glass–ceramics have been significantly improved. Furthermore, CaF2 promotes glass crystallization which does not inhibit the glass–ceramic bioactivity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Since the Bioglass® 45S5 (Na2O–CaO–SiO2–P2O5) was firstly reported by Hench et al. [1], many types of bioactive glasses and glass–ceramics are under development. At the same time, a novel research area on bioactive materials for bone repair and substitution has been developed [2–5]. The bioactive behavior of these glasses can be due to the formation of apatite-like layer, and the composition of apatite-like layer is equivalent to the mineral phase in bone [6]. However, most of bioactive glasses have a less flexural strength and fracture toughness and possess greater elastic modulus [7, 8], which means that the mechanical strength of these bioactive glasses is so insufficient that they can not used in load bearing applications.
Nowadays, many techniques for preparing bioactive glasses have been used in experiment research. Compared with traditional melting method, sol–gel method allows us to obtain glasses with higher purity, more specific surface area and inherent porous nature at a lower reaction temperature [9–11]. So sol–gel method attracts more interest and provides a more maneuverable way to synthesize bioactive glasses. However, some literatures showed that because the bioactivity of sol–gel derived biomaterials is so high, when they were applied to clinic, the mechanical properties would be decreased [12]. Therefore, it is necessary to optimize their properties to possess high bioactivity along with appropriate mechanical properties. Many factors such as chemical composition, structure, surface morphology, crystallinity and crystal size can make great impact on mechanical properties and in vitro bioactivity of bioactive glasses. Some researchers have tried to study the relationship between these factors and the properties of glasses [13–16]. The properties of bioactive glasses are mainly dependent on Si–O network. Mechanical properties and bioactivity can be controlled by modifying the network of the glasses with addition of different elements [17].
In the past decades, the Na2O–CaO–SiO2–P2O5 (45S5) glass–ceramics have been widely studied, and 45S5 bioglass is the most famous among these system because of its excellent bioactivity and biocompatibility. The low mechanical strength of 45S5 bioglass restricts its further clinical application, so it is necessary to modify 45S5 bioglass by changing its composition, structure, crystallinity and so on. In order to obtain bioactive sol–gel glass ceramics with the simplest composition, high mechanical strength and excellent bioactivity, a new Na2O–CaO–SiO2–P2O5 glass–ceramics by partial substitution of CaF2 for CaO was synthesized in this paper. The crystallization process and the mechanical and biological properties of synthesized glass–ceramic were studied, and the influence of the chemical composition on the structural features and bioactive properties was analysed.
2 Experimental
2.1 Synthesis
Two series of glasses in the Na2O–CaO–CaF2–SiO2–P2O5 system were prepared using the sol–gel method. Table 1 shows the nominal composition of the synthesized glasses and Table 2 shows the mass of raw materials of producing 100 g basic glass. Glasses were prepared by hydrolysis and polycondensation of appropriate amounts of tetraethyl orthosilicate (TEOS), triethyl phosphate(TEP), calcium nitrate (Ca(NO3)2·4H2O), NaNO3 and CaF2 in deionized water [mol of H2O/(mole of TEOS + mole of TEP) = 14]. Nitric acid (2 N) was used as catalyzer with a molar ratio of HNO3/(TEOS + TEP) = 0.06. The synthesis procedure was as follows: TEOS was mixed with water and HNO3, then TEP, Ca(NO3)2·4H2O, NaNO3 and CaF2 (if required) were successively added under continuous stirring by keeping 0.5 h intervals between additions. The sol was introduced into polyethylene containers at room temperature till the gel was formed. Then the gel was aged at 60 °C for 3 days, dried at 120 °C for 5 h and ball milled to obtain the powders with sizes ranging from 38 to 74 μm. After that powders were stabilized at 600 °C for 2 h. The obtained glass powder and appropriate amount of 7 wt% polyvinyl alcohol were mixed and granulated, and then put into the corresponding mold under 200 MPa using cold isostatic molding to prepare two kinds of compact samples. The first one is cuboid of 4 mm × 5 mm × 41 mm used to study mechanical property, the second one is disc of Ф10 mm × 2 mm used to study the biological activity. Finally, the compact samples were sintered in high temperature furnace with a heating rate of 3 °C min−1 according to the suitable heat treatment, which was determined by thermogravimetric and differential thermal analysis (TG/DTA) of the dried gel, then the furnace was naturally cooled down and glass–ceramics were obtained.
2.2 Characterization
The thermogravimetric and differential thermal analysis (TG/DTA) were carried out in SDT Q600 of TA Instruments from room temperature to 1,100 °C with a 10 °C min−1 heating rate under an air atmosphere. X-ray diffraction (XRD) patterns were recorded in a Bruker D8 Advance X-ray diffractometer using CuKα radiation (κ = 1.5406 nm) produced at 40 kV and 40 mA, and the range of diffraction angles (2θ) was scanned between 10° and 70° with a scan rate of 4° min−1 and a step size of 0.02°. Scanning electron microscopy (SEM) observations and energy dispersive spectrometer (EDS) analysis were carried out on JEOL JSM6380LA scanning. They were examined using an accelerating voltage of 20 kV and samples were coated with a film of gold before tests. Fourier-transform infrared (FTIR) spectroscopy analyses were made on a Bruker Optics VERTEX-70 FTIR spectrometer using KBr pellets in the range of 300–2,000 cm−1 with a transmission mode. The resolution used was 4 cm−1 and the number of scans was 32. The preparation of the KBr pellet was as follows: The testing sample powder (1 mg) and KBr powder (100 mg) dried in infrared lamp were fully mixed and milled in an agate mortar, and then put 80 mg mixture into the corresponding mold in tableting machine under 20 MPa lasting 1 min to prepare a 10 mm diameter KBr pellet. The volume density and apparent porosity of sintered sample were measured by the drainage method according to Archimedes principle, and the number of trials was 3. The bending strength and elasticity modulus were obtained in RGD-5 type electronic tensile machine by three-point bending method. The experimental results were means of 5 tests. The microhardness was determined by indentation test on the HV-1000 Huayin micro hardness tester. The load was 500 g and loading time was 15 s. Test number was 8, and the test results were means of the values, not including the minimum and maximum.
2.3 In vitro assays in SBF
In vitro assays were performed in a simulated body fluid (SBF), proposed by Kokubo et al. [18]. The ion concentration of the SBF solution shows in Table 3 and it is similar to inorganic parts of human plasma. During soaking process, the simulated body fluid contained in a polyethylene bottle was drawn in accordance with VSBF/SA = 10 cm, where SA was the total surface area of the soaked specimen. These bottles were covered with a tight lid and placed in a water bath with constant temperature at 37 °C, meanwhile, the SBF solution was continuously replaced every 2 days. After being soaked, samples were rinsed with deionized water and acetone and then dried in air at room temperature. The formation of an apatite-like layer on the samples surface was determined by XRD, SEM, EDS and FTIR analysis as described above.
3 Results and discussion
3.1 Thermal property analysis
Figure 1 shows the TG/DTA curves of the gels with different component after being dried at 120 °C. From the TG plots, there were two pronounced weight loss processes. The initial one was observed from room temperature to 272.69 and 267.41 °C and the weight loss were both 15 %. It should be attributed to the loss of residual water and ethanol. The second one was terminated at 727.14 and 764.53 °C, with a weight loss corresponds to 54 and 49 %, respectively, which might be caused by the loss of nitrates used in the experiment. Thereafter, slightly changes took place during the rest time.
The specific heat capacity of material will change around the glass transition temperature (Tg), meanwhile, there will appear the turning point of endothermic direction and sometimes small endothermic peak will also appear on DTA curve, which can be used to determine the glass transition temperature [19]. From the DTA curve, it was observed that the glass transition temperature (Tg) corresponded to 517.22 and 520.90 °C respectively. In addition, the exothermic peak denoting the emergence of crystallization were detected in DTA plots. The crystallization peaks of 45S5 sample were observed at 709.83 and 847.24 °C, and the sample 45S5-F was observed at 688.58 and 808.92 °C. According to the results of TG/DTA, we selected 600 °C as the stabilization temperature. The chosen temperature was below the crystallization temperature, but nitrates were not completely eliminated at this temperature.
The crystallization process of the sol–gel derived glass has two steps, including nucleation and crystal growth, so microcrystal glass–ceramic can be obtained by heating for a certain period of time at nucleation temperature and crystallization temperature, respectively. The literature shows that nucleation temperature should be generally tens of degrees higher than the glass transition temperature (Tg), and crystallization temperature is equal to the exothermic peak temperature [20]. The exothermic crystallization peaks of different components of glass essentially appear near the 710 and 850 °C, which were determined as crystallization temperature. The glass transition temperature (Tg) was near 520 °C, so 550 °C was chosen as nucleation temperature. In this paper, in order to study the effect of the partial substitution of CaF2 for CaO on glass structure, performance and bioactivity, a unified system of heat treatment which is shown in Fig. 2 has been used. That is heating the glasses 30 min at 550 °C for nucleation followed by 2 h at 710 °C and 1 h at 850 °C for crystal growth with the heating rate of 3 °C min−1, then the furnace was naturally cooled down.
3.2 Characterization of glass
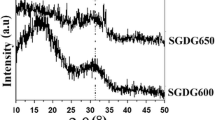
Figure 3a is the XRD patterns of two samples before crystallization treatment. The two XRD patterns were similar and crystal NaNO3 was detected. The existence of NaNO3 is because that the stabilization temperature 600 °C is below the temperature that NO3 − is fully decomposed. Both samples showed the characteristic of amorphous material with a wide band at 20–38°(2θ), indicating the glass powder after stabilization is mainly amorphous. Besides, some small diffraction peaks of Na2CaSi3O8 were observed in the sample 45S5-F, which means that CaF2 can promote crystallization of the initial glass. Trace amounts of Ca3Si2O7 likely due to the combination of CaO and SiO2 were also detected. The radius of F− (1.36 Å) is very close to that of O2− (1.40 Å), so F− could replace O2− easily. When CaO was partly replaced by CaF2, according to the electrostatic valence equilibrium rules, double F− could substitute an O2− in the glass network structure and the bonds in ≡Si–O–Si≡ network would be broken [21], which make the original large silicone atom divide into smaller groups. Consequently, structural rearrangement potential barrier is reduced and crystallization tendency is increased. Furthermore the fracture of the glass network structure reduces the stability of the glass. The thermal analysis results (Fig. 1) also confirm that CaF2 promotes crystallization because temperature corresponding to the exothermic crystallization peaks of the CaF2 doped glass is lower than that of the glass without CaF2.
The XRD patterns of the sample sintered according to heat treatment procedure are shown in Fig. 3b. NaNO3 was not observed in the XRD results, which is caused by the fact that the crystallization temperature is above the temperature that NO −13 is fully decomposed. It was worth noting that amorphous diffraction band disappeared and some new sharp crystal peaks appeared, indicating crystallization. Some patterns were consistent with the standard XRD patterns for Na6Ca3Si6O18 (JCPDS77-2189), Na4Ca4[Si6O18] (JCPDS75-1687, 79-1084, 1086, 1087, 1088, 1089, 78-0364), Na4.24Ca3.8[Si6O18] (JCPDS78-1649). The crystalline phase is not a single substance, but the structures of the above crystals are similar. The chemical formula of all the crystalline phases can be denoted as NaxCa6–0.5xSi6O18, where X ranges from 4 to 6, and Na6Ca3Si6O18 was the principal phase. In addition, the peaks of Na2CaSi3O8 were detected in the two glasses. Compared the intensity of the diffraction peaks on the two patterns, it can be seen that the diffraction peaks of 45S5-F sample was more strong. In Fig. 3b, the crystallinity of the 45S5-F sample was 4.60 % higher than that of 45S5 sample calculated by jade 5.0, which means that the partial substitution of CaF2 for CaO can promote the glass–ceramic crystallization. This is consistent with some literatures, which suggested that when the sample being crystallized, F− firstly dissolves out of the melt, provides nucleation position for crystal precipitation and then promotes crystallization [22].
3.3 Performance test results
After crystallization treatment, the evaluations of the textural and mechanical properties were conducted and the results are shown in Table 4. It is clear to see that the substitution of CaF2 for CaO has remarkable influence on glass volume density, apparent porosity, bending strength, elasticity modulus and microhardness. It should be attributed to the relative molecular mass and bond energy. The relative molecular mass of CaF2 is larger than CaO and Ca–F bond energy is larger than Ca–O. So when CaO was substitute by CaF2, the density and strength of glass will increase. On the other hand, CaF2 promotes crystallization, makes the crystal content increase, also leads to the glass density increasing.
3.4 In vitro assays
Figure 4 shows the XRD patterns of the two glass–ceramics after different soaking time in SBF solution. After soaking in SBF for 2 days, the characteristic peaks of carbonated hydroxyapatite (Ca10(PO4)3(CO3)3(OH)2) were obviously found but not sharp. The obvious broad humps peak for amorphous was found, meanwhile, the matrix phase Na2CaSi3O8 appeared in the diffraction pattern of the samples. Besides, a tiny amount of diffraction peaks Ca10(PO4)6(OH)2 appeared on the surface of 45S5 sample. When glass–ceramics were soaked in SBF for 7 days, the characteristic peaks of carbonated hydroxyapatite Ca10(PO4)3(CO3)3(OH)2 was obviously increased and the diffraction intensity of the matrix phase Na2CaSi3O8 were significantly reduced. The appearance of Na2CaSi3O8 in the XRD patterns is caused by the fact that the precipitate on the surface of the sample is thin, therefore, X ray can penetrate precipitate and detect the glass matrix. In addition, the 45S5 sample showed the characteristic of amorphous material at 20–40°(2θ). When CaF2 partly substitutes CaO, the broad humps peak for amorphous did not existed and the peak of Ca10(PO4)3(CO3)3(OH)2 was very sharp, which shows the crystallinity of precipitate is higher than 45S5. The appearance of carbonated hydroxyapatite Ca10(PO4)3(CO3)3(OH)2 is similar to the conclusion of Peitl et al. [23], they suggested that carbonated hydroxyapatite layer can be formed when glass–ceramic were soaked in SBF, even the glass–ceramic has been fully crystallized. Some scholars has proved that the glass after crystallization can reduce its biological activity [24]. But, crystallization did not inhibit the bioactivity of samples in this research. After soaking in SBF for 14 days, the matrix phase Na2CaSi3O8 practically disappeared and the diffraction peaks of hydroxyapatite also appeared on the sample 45S5-F. And, with the increasing of immersion time, the diffraction intensity of hydroxyapatite and carbonated hydroxyapatite increased. Besides, when the two samples soaked the same time in SBF, the diffraction intensity of apatite in Fig. 4b was more strong than that in Fig. 4a, which shows that the sample doped with F− has better bioactivity than the sample 45S5.
Figure 5 shows the FTIR spectra of 45S5-F glass–ceramics before and after soaking in SBF. The stretching vibration peak at 1,644 cm−1 denoting -OH due to the deformation mode of H–O–H was observed. The band around 1,487, 1,413, 1,041 and 695 cm−1 respectively assigned to O–Ca–O, Na-OH, P–O(s) and Si–O–Ca were also observed. Besides, the vibration peak at 1,727 and 520 cm−1 corresponding to Si–O–Na+ also appeared on the glass. Silicate absorption bands at 1,085, 800 and 464 cm−1, which are respectively assigned to the asymmetric stretching mode Si–O(s, asym), the symmetric stretching vibration Si–O–Si(s, sym) and the rocking vibration Si–O(r), were observed on the spectra of the glasses after crystallization. In addition, the Si–O–NBO group was found at 910 cm−1. Calcium oxide can insert into glass structure, such as, ≡Si–O–Si≡ + CaO → ≡Si–O−…Ca2+…−O–Si≡, induce the disruption of bridging oxygen bonds and lead the formation of NBO bonds. Some researches have demonstrated that the ion exchange is affected by Si–O–NBO bonds in the glass network. An efficient ion exchange, dissolution of the silica and formation of a SiO2-rich layer on the surface required a certain amount of Si–O–NBO [25–27]. The double peaks at 572 and 608 cm−1 were assigned to the bending vibration P–O(b) related to the presence of crystalline phosphate in the glasses. However, this two peaks seem to show a contradiction with the results obtained by XRD (Fig. 3b), which can be explained by the fact that the technology of FTIR probes short-range structural order, whereas XRD reveals long-range order in the glass structure. This similar phenomenon was also detected by Vallet-Regí et al. [28]. They suggest that a crystalline layer may formed on the surface of the glass under atmospheric conditions because that the glass samples in powder form have high surface area and high bioactivity. Furthermore, the nucleation of such a layer is likely to be due to the reaction between the atmospheric water and Ca2+ ions and phosphate groups contained in the glass composition.
After soaking in SBF for 2 days, the two peaks related to Si–O–Na+ disappeared, and the double peaks P–O(b) transformed into P–O(cryst). Meanwhile, Ca–O–Ca and Na-OH disappear, and C–O(s) was detected. It is worth noting that Si–O–Ca, Si–O–NBO, Si–O(s) and Si–O(r) gradually weakened and when after soaking in SBF for 7 days, they disappeared, namely that the precipitate covered on the sample surface. With the extension of soaking time, the vibration frequencies of Si–O-Si(s, sym) basically firstly increased, then decreased. And the peak value at 1,041 cm−1 corresponding to the stretching vibration peak of P–O(s) firstly decreased, then increased and finally maintained a fixed value. The reason of this phenomenon is that Ca2+ in initial glass firstly degradates very fast, then gradually achieves balance between degradation and sedimentary of Ca2+ in the SBF. The paired phosphate absorption bands of P–O(cryst) corresponding to PO4 3− gradually increased, indicating the formation of apatite. The bending vibration peak around 871 cm−1 corresponding to C–O(b) gradually increased, indicating the formation of carbonate apatite [29, 30]. It is known that the apatite-like layer is formed from a large number of apatite nucleuses, then followed by the growth of spherical clusters. The process of apatite formation on bioactive glass surface is complex and involves many reactions, which was studied by Hench et al. [23]. They proposed that a rapid ion exchange between Na+ or Ca2+ of material and H+ or H3O+ in the solution took place, which lead to the formation of SiO2-rich layer on glass surface, then Ca2+ and PO4 3− were migrated onto SiO2-rich layer followed by nucleation and growth of apatite [31]. Moreover, the vibrational peak of Si–O–Na+, Si–O–NBO and Si–O–Ca disappeared or weakened and the new vibration peak of P–O and C–O emerged and strengthened with the increasing immersion time, which also indicating that the sample experiences a dissolution-precipitation. The sample after soaking in SBF for 2 days generates a apatite layer, which illustrates that the crystallized glass prepared in this experiment has a high biological activity.
The appearance of the phosphate and carbonate absorption bands in the glass spectra after soaking in SBF confirms that the newly formed material contains carbonate hydroxyapatite (CHA). Carbonate hydroxyapatite is a kind of biological material and has been reported to have better biological activity than pure hydroxyapatite. Hydroxyapatite (HA) is identified as the least soluble and the most stable material among the calcium phosphates. Thus it has undesirable characteristic because HA may impede the rate of bone regeneration on implantation. When carbonate is incorporated into HA, carbonate can cause an increase in solubility, change in the crystal morphology and better biological activity [32]. CO3 2− ions can replace both the A site (a substitution of CO3 2− for OH−) and the B site (a substitution of CO3 2− for PO4 3−) in the apatite structure. In the A site, the peaks of CO3 2− appear at 880, 1,450–1,455 and 1,540–1,550 cm−1. In the B site, the peaks of CO3 2− appear at 860–870, 1,410–1,420 and 1,455–1,470 cm−1. It has been proved in the literature [33] that CO3 2− substitution at the A site in apatite crystals is limited to the preparation in water system. In this research, the C–O peaks denoting CO3 2− shown in Fig. 5 are assigned to the B site, where a substitution of CO3 2− for PO4 3− is conducted.
Figure 6 shows the morphologies of the 45S5-F glass–ceramics after different soaking time in SBF solution. As shown in Fig. 6a, the surface of glass–ceramics doped with CaF2 was compact and smooth. At a soaking time of 2 days, the morphology of glass surface changed distinctly compared with the initial glass surface. The glass surface was partially covered by some lath-shaped, acicular and spherical particles, but the particles were not sufficient to cover the entire surface and the initial glass surface was still observed (Fig. 6b). After soaking for 7 days, aggregation was observed in some zones (Fig. 6c). With the extension of soaking time, the glass surface was fully covered by apatite layer and spherical agglomerates was more apparent. Especially, the surface of the sample soaked for 21 days was more compact and covered with the uniform size of particles.
Figure 7 shows the variation of Ca/P molar ratio of the precipitates formed on the 45S5-F glass–ceramics surface after soaking in SBF according to the results of the EDS analysis. With the prolonging of soaking time, the Ca/P ratio of the precipitates gradually reduced. Before immersion in SBF, sample surface was rich in calcium and depleted in phosphorus, and the Ca/P ratio was 4.05. The Ca/P ratio was slightly higher than that of 4.02 in raw materials, which reason is that a trace amount of phosphorus volatilized during heat treatment. In the beginning of immersion time, Ca2+ dissolved and then precipitated on the sample surface, and after that the apatite layer generated on the surface, which results in the decrease of the Ca/P ratio. When the sample soaked in SBF for 21 days, the Ca/P ratio of the precipitates on the surface reduced to 1.71, which is close to the theoretical Ca/P ratio (1.67) of the hydroxyapatite.
4 Conclusions
Na2O–CaO–SiO2–P2O5 system ceramics, in which CaO is partly replaced by CaF2, have been obtained by the sol–gel method, and the thermogravimetric and differential thermal analysis results show that the crystallization process of the glass–ceramic can be conducted by a suitable heat treatment procedure. The crystalline glass–ceramic is composed of the phases of NaxCa6-0.5xSi6O18 and a little Na2CaSi3O8, and partial substitution of CaF2 for CaO promotes glass crystallization. The presence of CaF2 can obviously increase the volume density, bending strength and microhardness of the glass and decrease its apparent porosity. The in vitro assays results show that the obtained glass–ceramic has good bioactivity, and crystallization does not inhibit the glasses bioactivity. With the increasing of soaking time, the Ca/P ratio of apatite gradually reduces. When the sample soaked in SBF for 21 days, the Ca/P ratio is close to the theoretical Ca/P ratio of the hydroxyapatite.
References
Hench LL, Splinter RJ, Allen WC, Greenlee TK (1971) Bonding mechanisms at the interface of ceramic prosthetic materials. J Biomed Mater Res 5:117–141
Cabal B, Malpartida F, Torrecillas R, Hoppe A, Boccaccini AR, Moya JS (2011) The development of bioactive glass–ceramic substrates with biocide activity. Adv Eng Mater 13:B462–B466
Padmanabhann SK, Gervaso F, Carrozzo M, Scalera F, Sannino A, Licciulli A (2013) Wollastonite/hydroxyapatite scaffolds with improved mechanical, bioactive and biodegradable properties for bone tissue engineering. Ceram Int 39:619–627
Vitale-Brovarone C, Nunzio SD, Bretcanu O, Vern E (2004) Macroporous glass–ceramic materials with bioactive properties. J Mater Sci Mater Med 15:209–217
Martorana S, Fedele A, Mazzocchi M, Bellosi A (2009) Surface coatings of bioactive glasses on high strength ceramic composites. Appl Surf Sci 255:6679–6685
Singh VK, Reddy BR (2012) Synthesis and characterization of bioactive zirconia toughened alumina doped with HAp and fluoride compounds. Ceram Int 38:5333–5340
Simon V, Lucacel RC, Titorencu I, Jinga V (2011) Physical properties and biological performance of bioactive glasses and glass–ceramics tested in vitro. Key Eng Mater 1463:85–89
Soares P, Laurindo CAH, Torres R, Kuromoto N, Peitl O, Zanotto E (2012) Effect of a bioactive glass–ceramic on the apatite nucleation on titanium surface modified by micro-arc oxidation. Surf Coat Technol 206:4601–4605
Saravanapavan P, Hench LL (2000) Bioactive sol–gel glasses in the CaO–SiO2 system. Key Eng Mater 416:609
Chatzistavrou X, Tsigkou O, Aminb HD, Paraskevopoulos KM, Salih V, Boccaccini AR (2012) Sol–gel based fabrication and characterization of new bioactive glass–ceramic composites for dental applications. J Eur Ceram Soc 32:3051–3061
Chen XF, Meng YC, Yu LL, Naru Z (2008) Investigation on bio-mineralization of melt and sol–gel derived bioactive glasses. Appl Surf Sci 255:562–564
Ma J, Chen CZ, Wang DG, Meng XG (2010) In vitro degradability and bioactivity of mesoporous CaO–MgO–P2O5–SiO2 glasses synthesized by sol–gel method. J Sol–Gel Sci Technol 54:69–76
Castillo J, Yanes AC, Méndez-Ramos J, Velázquez JJ, Rodríguez VD (2011) Structural and luminescent study in lanthanide doped sol–gel glass–ceramics comprising CeF3 nanocrystals. J Sol–Gel Sci Technol 60:170–176
Assem EE (2005) Effect of replacing calcium oxide with calcium fluoride on some physical properties of borosilicate glass ceramics. J Phys D Appl Phys 38:942–945
Sanada T, Seto H, Morimoto Y, Yamamoto K, Wada N, Kojima K (2010) Luminescence and long-lasting afterglow in Mn2+ and Eu3+ co-doped ZnO–GeO2 glasses and glass ceramics prepared by sol–gel method. J Sol–Gel Sci Technol 56:82–86
Ghasemzadeh M, Nemati A, Baghshahi S (2012) Effects of nucleation agents on the preparation of transparent glass–ceramics. J Eur Ceram Soc 32:2989–2994
Guo HW, Gong YX, Gao SY (2010) Preparation of high strength foam glass–ceramics from waste cathode ray tube. Mater Lett 64:997–999
Kokubo T, Kushitani H, Sakka S, Kitsugi T, Yamamuro T (1990) Solutions able to reproduce in vivo surface-structure changes in bioactive glass–ceramic A-W. J Biomed Mater Res 24:721–734
Liu ZH (1994) Volume VI of the handbook of analytical chemistry. Chemical Industry Press, Beijing
Hong Z, Reis RL, Mano JF (2009) Preparation and in vitro characterization of novel bioactive glass ceramic nanoparticles. J Biomed Mater Res 88:304–313
Pacurariu C, Lazau RI, Lazau I, Ianos R, Tita B (2009) Non-isothermal crystallization kinetics of some basaltic glass–ceramics containing CaF2 as nucleation agent. J Therm Anal Calorim 97:507–514
Atiar RM, Bikram JB (2009) Microstructure, mechanical, and in vitro properties of mica glass–ceramics with varying fluorine content. J Mater Sci Mater Med 20:869–882
Peitl O, Zanotto ED, Hench LL (2001) Highly bioactive P2O5–Na2O–CaO–SiO2 glass–ceramics. J Non Cryst Solids 292:115–126
Mukherjee DP, Das SK (2013) SiO2–Al2O3–CaO glass–ceramics: effects of CaF2 on crystallization, microstructure and properties. Ceram Int 39:571–578
Serra J, Gonzalez P, Liste S, Chiussi S (2002) Influence of the non-bridging oxygen groups on the bioactivity of silicate glasses. J Mater Sci Mater Med 13:1221–2122
Brauer DS, Anjum MN, Mneimne M, Wilson RM, Doweidar H, Hill RG (2012) Fluoride-containing bioactive glass–ceramics. J Non Cryst Solids 358:1438–1442
Jallot E, Benhayoune H, Kilian L, Irigaray JL, Balossier G, Bonhomme P (2000) Growth and dissolution of apatite precipitates formed in vivo on the surface of a bioactive glass coating film and its relevance to bioactivity. J Phys D Appl Phys 33:2775–2780
Arcos D, Greenspan DC, Vallet-Regí M (2002) Influence of the Stabilization temperature on textural and structural features and ion release in SiO2–CaO–P2O5 sol–gel glasses. Chem Mater 14:1515–1522
Izquierdo-Barba I, Salinas AJ, Vallet-Regí M (2000) Effect of the continuous solution exchange on the in vitro reactivity of a CaO–SiO2 sol–gel glass. J Biomed Mater Res 51:191–199
Attwood D, Mosquera V, Rey C, Garcia M (1991) Temperature dependence of molar volume and adiabatic compressibility of aqueous solutions of an amphiphilic phenothiazine drug. J Colloid Interface Sci 147:316–320
Ma J, Chen CZ, Wang DG, Meng XG, Shi JZ (2010) Influence of the sintering temperature on the structural feature and bioactivity of sol–gel derived SiO2–CaO–P2O5 bioglass. Ceram Int 36:1911–1916
Landi E, Celotti G, Logroscino G, Tampieri A (2003) Carbonate hydroxyapatite as bone substitute. J Eur Ceram Soc 23:293–2937
Sato K, Kogure T, Kumagai Y, Tanaka J (2001) Crystal orientation of hydroxyapatite induced by ordered carboxyl groups. J Colloid Interface Sci 240:133–138
Acknowledgments
This work was financially supported by the Department of Science and Technology of Shandong Province (Grant No. 2009GG10003032).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Li, H.C., Wang, D.G., Hu, J.H. et al. Crystallization, mechanical properties and in vitro bioactivity of sol–gel derived Na2O–CaO–SiO2–P2O5 glass–ceramics by partial substitution of CaF2 for CaO. J Sol-Gel Sci Technol 67, 56–65 (2013). https://doi.org/10.1007/s10971-013-3050-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-013-3050-5