Abstract
The surface of magnetite nanoparticles was coated with functional polysiloxane layers using reaction of hydrolytic copolycondensation of tetraethoxysilane and 3-aminopropyltriethoxysilane (or N-[3-trimethoxysilylpropyl]ethylendiamine), and also that of tetraethoxysilane, 3-aminopropyltriethoxysilane and methyltriethoxysilane (or n-propyltriethoxysilane). It was shown that these functionalized magnetically controllable particles (about 60–150 nm in size as aggregates), as opposed to magnetite, adsorb urease well from aqueous solutions (up to 1 g/g), and that the level of residual activity of adsorbed layers is up to 84 % in the case of a bifunctional sample. It was established that the activity of immobilized urease is normally gradually reduced during storage of the samples, but in the case of ethylenediamine functional group is not decreased for 45 days. The synthesized samples are promising for use as magnetically directed biocatalysts.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Due to the continuously increasing use of immobilized enzymes in biocatalysis, biotechnology, and for production of new drugs [1–5], this area is currently enjoying an increased interest. Both the class of applied enzymes and the carriers for their fixation are diversifying. Polysiloxane matrices obtained by hydrolytic sol–gel method are very promising for enzyme binding. Their primary advantage is high adsorption capacity [6]. In addition, their functional characteristics such as parameters of the porous structure, composition of the surface layer, the degree of hydrophobicity, etc. can be adjusted in the synthesis of such carriers. This allows to achieve an efficient immobilization of enzymes [7, 8]. In other words, the immobilized enzymes retain a high degree of activity, and their consumption is minimized. However, the literature data concerning the activity of enzymes adsorbed on silica matrix are rather contradictory. It is assumed that the silanol groups on the surface of silica adsorbents are involved in binding of the enzymes and, dependent on the pH, such binding can occur either via proton transfer mechanism, or through formation of the hydrogen bonds [9]. It has also been shown that the adsorption of enzymes on carriers with both hydrophobic and hydrophilic surface may be irreversible, dependent on the process conditions and the nature of the surface layer of the carrier [10, 11]. In general, the trends in protein adsorption are determined mainly by the porous structure of the matrix and the nature of the surface layer.
Special interest recently has been revealed to magnetically directed sorbents, primarily the magnetite derived ones. It turned out that, using hydrolytic polycondensation reactions with alkoxysilane precursors it is possible to deposit on the surface of magnetic nanoparticles a polysiloxane layer incorporating the functional groups required for fixation of enzymes. In addition, introduction into this layer of organic functional groups can create for an enzyme an environment resembling the intracellular medium [8, 10–13]. Moreover, the creation of the polysiloxane layer on the surface of magnetite particles and the enzyme immobilization can be carried out simultaneously using the above-mentioned reaction. This approach opens opportunities to approach a new generation of composite biocatalysts, combining the advantages of silica supported enzymes with the easy directability and facile handling typical of magnetite [8, 12–15].
Therefore, the aim of the present work was to evaluate the simplest approach to obtaining magnetically controlled biocatalysts based on urease, namely the adsorption of the enzyme on the surface of functionalized nanoparticles of magnetite. In this case, the reaction of the hydrolytic polycondensation in two- and three-component (for alkoxysilanes) systems, respectively, was used to create a functional layer containing amine, ethylenediamine and amine/alkyl groups.
2 Experimental
The applied reagents involved tetraethoxysilane, Si(OC2H5)4 (TEOS, Aldrich, 98 %); 3-aminopropyltriethoxysilane, (C2H5O)3Si(CH2)3NH2 (APTES, Aldrich, 99 %); methyltriethoxysilane, (C2H5O)3SiCH3 (MTES, 99 %, Aldrich); n-propyltriethoxysilane, (C2H5O)3Si(CH2)2CH3 (PTES, 97 %, Fluka); N-[3-trimethoxysilylpropyl]ethylendiamine, (CH3O)3Si(CH2)3NH(CH2)2NH2 (TMPED, 97 %, Aldrich); ethanol (96 %); acetone (“Macrochem”, Ukraine); NH4F (98 %,“Reachem”, Ukraine); 0.1 M HCl, 0.1 M NaOH, and 0.1 M EDTA were prepared using fixanal concentrates (Cherkasy State Chemical Plant, Ukraine); for 0.06 M phosphate buffer (pH 7.0) were used Na2HPO4·2H2O and KH2PO4 (“Macrochem”, Ukraine); 2 M urea solution (“Macrochem”, Ukraine, analytical grade). Urease used in work belonged to the class of hydrolases, and was derived from soybeans “Jack beans” (EC 3.5.1.5, activity 26.7 U/mg (pH 7), Fluka).
Magnetite was prepared by co-precipitation from salts of iron (II) and (III) with ammonia in argon atmosphere [16]. The obtained magnetic particles are spherical. Their average diameter is close to 14 nm and the specific surface area is ~90 m2/g.
Deposition of a functional layer on the surface of magnetite particles has been described in detail by us in [17, 18]. Here we present a brief procedure. Sample batch of Fe3O4 was treated by ultrasound for 10 min in 45 cm3 of distilled water. APTES (or TMPED), dissolved in 4 cm3 of ethanol, and 1.25 cm3 of 1 % NH4F solution in water were added to the resulting suspension that was stirred then for 5 min. Then slowly, drop by drop, TEOS was added to achieve the ratio of reactants Fe3O4/TEOS/APTES (TMPED) = 0.0022/0.034/0.0059. For preparation of bifunctional coatings each trifunctional silane was dissolved separately in ethanol, a catalyst was added to the solution and it was then poured into a suspension of magnetite in water one after another to achieve the required ratio of the components Fe3O4/TEOS/APTES/MTES (PTES) = 0.0022/0.034/0.0028/0.0028. Stirring was continued for 6 h at room temperature. Dark brown precipitate was separated by a magnet, washed with water (3 × 50 cm3), ethanol (2 × 50 cm3), and acetone (2 × 50 cm3) and dried in air.
The resulting samples were labeled as follows: Fe3O4/SiO2/APTES—1, Fe3O4/SiO2/APTES/MTES—2, Fe3O4/SiO2/APTES/PTES—3, Fe3O4/SiO2/TMPED—4.
The DRIFT spectra were recorded on the Thermo Nicolet Nexus FT-IR at 8 cm−1 resolution using the Spectra Tech collector diffuse reflectance accessory at room temperature. The samples were mixed with KBr (1:30) and were used to fill the DRIFT sample cup before measurements.
The nitrogen adsorption isotherms for all the samples were measured on a “Kelvin-1042” adsorption analyzer (Costech Microanalytical). Before the measurements, the samples were out gassed at 383 K in the helium atmosphere. The BET specific surface area [19] was calculated in the relative pressure range between 0.05 and 0.35.
Morphology of the obtained samples was studied by JSM-6060LA Analytical Scanning Electron Microscope (Jeol, Tokyo, Japan) using secondary electrons at accelerating voltage of 30 kV. For SEM studies, the samples were fixed on the surface of object table. To prevent the accumulation of the surface charges and improve the contrast, the surface of the samples was covered with thin continuos layer of gold or platinum by cathodic sputtering in vacuum. For textural investigations, the samples were deposited on a copper-grid-supported transparent carbon foil and examined by a conventional transmission electron microscopy TEM/HRTEM (JEOL JEM 2010-Fx) operating at 200 kV and TEM JEOL JEM 1230. Elemental analysis of the prepared samples using an Energy-dispersive X-ray spectroscopy (EDS) was also performed in combination with transmission electron microscopy.
The content of amino groups was determined by acid–base titration. Batches of the samples (0.1 g) were treated with a 0.1 M HCl solution (20 cm3) for 24 h. The precipitates were removed by a magnet and the filtrate was titrated with 0.1 M NaOH in the presence of the indicator (methyl orange). Concentration of the amino groups was determined from the difference between the content of protons in solution before and after sorption.
Urease enzyme activity was determined by the rate of formation of ammonia in the urea hydrolysis reaction at 25 °C [20]. In all cases, the activity was assumed as the average of three parallel experiments, the biggest difference between them did not exceed 10 %. The average error in urease activity determined taking into account the Student’s coefficient for reliable probability 0.95 did not exceed 10 %.
To study the kinetics of urease adsorption 0.05 g functionalized magnetite samples were shaken in a test tube with 2 cm3 urease buffer with the concentration of 2.5 g/L. The solution above the precipitate was analyzed for enzyme after 5, 10, 20, 30 and 60 min. In all cases, the amount of bound enzyme was estimated from the difference between the urease taken for immobilization and found in the solution. The content of urease in solution after adsorption and in the rinse waters was determined from its activity in relation to the specific activity of the enzyme. It was assumed that the specific activity of the immobilized enzyme was the same as for the native one.
Immobilization of urease on functionalized magnetite was performed by static adsorption method in a buffer solution with urease concentration 1.25–7.5 g/L. The adsorption experiments were carried out at room temperature for 4 h, with occasional stirring. A sample of functionalized magnetite (0.05 g) was covered with a solution of urease (5.0 mg) in 2 cm3 of phosphate buffer and EDTA (volume ratio 9:1). The precipitate was separated from the solution by a magnet and washed with phosphate buffer 5 times using 5 cm3 aliquotes. Maximal adsorption of urease was calculated in this case as the difference between the urease taken for immobilization and the one found in solution.
Langmuir constants were calculated from the adsorption isotherms using the formula (Fig. 4):
where Nc—concentration of the solute remaining in solution in the state of equilibrium (mmol L−1); Nf—the amount of solute adsorbed in the same condition (mmol g−1); Nf max—the maximum adsorption capacity in the monolayer and b—the equilibrium constant of the adsorption process [21].
3 Results and discussion
Hydrolytic polycondensation reaction using two- or three-component alkoxysilane systems was used for creation of the functional layer on the surface of magnetite. In this case, the product of hydrolysis and polycondensation of TEOS was acting as structuring agent and the trifunctional silane as the functionalizing one. Figure 1 shows the SEM micrographs of the samples. It demonstrates that all the obtained samples are composed of particles having spherical shape, but their sizes are significantly increased. Thus, for sample 1 the average diameter of the particles is about 100 nm, for sample 2—~60 nm, for sample 3—~150 nm, for sample 4—100 to 120 nm. It should be noted that in the case of sample 3, the particles are most probably aggregates of smaller particles. The TEM studies demonstrate that the particles displayed in the SEM images (Fig. 1) are not individual but are composed of aggregates of smaller ones (see Fig. 2). The cores of these particles are constituted of magnetite nanoparticles 10-20 nm in size (Fig. 2a, b). It has to be noted that these core particles are in turn often composed of several individual particles of magnetite (Fig. 2b). The polysiloxane shell of a “primary” particle incorporates thus several Fe3O4 particles simultaneously. Figure 2c,d,f shows that the produced samples are built up of such “primary” particles. In case of sample 3 containing 3-aminopropyl and n-propyl groups in the surface layer, these “primary” particles are less aggregated (see Fig. 2e). The same is characteristic also of the sample 4 (Fig. 2g). It is important to note that formation of the polysiloxane layer on the surface of the magnetite particles does not deteriorates their magnetic characteristics as it has been demonstrated earlier by us in the case of samples 1 and 4 [17]. The spectra obtained by EDS for sample 1 (Fig. 2g) have peaks for the C, N, O, Fe, Si atoms indicating the presence of 3-aminopropyl groups and polysiloxane layer on the surface of magnetite.
The specific surface area of the samples is in agreement with the abovementioned particle size. Thus, for samples 1 and 4 having similar particle diameters, the value of the specific surface area is 100 and 150 m2/g, respectively. For sample 2, formed by smaller particles, Ssp = 180 m2/g, while for the sample 3, it is 70 m2/g.
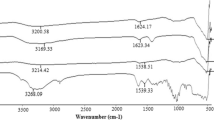
The presence of a polysiloxane layer and the functional groups on the surface of magnetite was confirmed by IR spectroscopy (Fig. 3). In the spectra of all the samples in the 1,000–1,200 cm−1 region was observed an intense and broad absorption band corresponding to νas(SiOSi) vibrations of the polysiloxane skeleton [22]. The band of medium intensity at 1,545–1,555 cm−1 in the IR spectra of the samples (in case of the sample 4, it has the form of a shoulder) refers to deformation vibration δ(NH2) of the aminogroup [23]. The sharp absorption band at 1,280 cm−1 visible in the IR spectrum of sample 2 obtained using MTES, can be attributed to the δs(CH3) of a methyl group attached to a silicon atom [23]. The IR spectra of all samples (Fig. 3) display also absorption bands at 550–650, 1,635–1,645 and 2,850–2,950 cm−1 which can be attributed to the ν(FeO), δ(H2O) and νs,as(CH) vibrations, respectively.
Thus, the synthesized samples consist of nearly spherical particles having a surface layer of polysiloxane bearing available amine groups. This is confirmed by acid–base titration, showing the content of amino groups in the sample 1 to be 1.8 mmol/g, 2—1.3 mmol/g, 3—1.7 mmol/g, 4—3.1 mmol/g (values close to those reported in [17]).
The kinetics of the urease adsorption was studied initially to assess the protein-binding properties of the samples (see Fig. 4). This figure shows that the adsorption equilibrium is achieved within 5 min for sample 3, 10 min for samples 1 and 2, and within 20 min for sample 4. No further growth of the sorption capacity was observed on increase of the interaction times. Somewhat longer time to achieve the enzyme adsorption equilibrium may be associated for the sample 4 with difficulties in diffusion caused by the large geometric dimensions of the functional group. In addition, one can not exclude that the ethylenediamine group is capable to form more hydrogen bonds in the surface layer of the matrix and the adsorption of urease requires then more time for its reorientation.
Figure 4 shows also highest degree of urease binding (98 %) for the samples of magnetite with alkyl groups in surface layer compared to the maximum degree of urease binding of only 65 % for the sample 4 (bearing ethylenediamine functional groups).
Excess adsorption isotherms of urease from buffer solution for functionalized magnetite samples are shown in Fig. 5. With the exception of the isotherm for the sample 4, they are similar in shape and display a sharp rise at low levels of urease in the initial solution, which may be indicative of a strong interaction between adsorbate and adsorbent. Further increase in the urease concentration in solution makes the curves to reach a plateau (Fig. 5). It is believed that such shape of isotherms is observed when the adsorption is not subject to strong competition with the solvent molecules, or when strong intermolecular interactions occur between the adsorbed molecules [4]. Perhaps both these factors play a role in the present case. The adsorbed urease is an enzyme with high molecular weight, which exists in aqueous solutions in the form of large particles aggregates due to strong interactions between the functional groups. We should therefore expect a multicenter adsorption, where the competition of water molecules is negligible.
The obtained urease adsorption isotherms (Fig. 5) are Langmuir type ones [24]. This indicates high affinity of urease molecules to the surface functional groups of the support, which enhances the adsorption of the enzyme. Figure 5 shows also that urease adsorption is significantly higher in the case of magnetite samples containing alkyl groups along with the aminogroups in the surface layer (samples 2 and 3) compared to sample 1 containing only 3-aminopropyl groups. Probably, the alkyl groups are shielding the 3-aminopropyl groups that are less likely then to form hydrogen bonds with silanol groups in this case. Consequently, these groups can be “easily” connected (in some way) to the surface groups of urease and this affects both the maximum adsorption of urease [25], and the kinetics of the process (Fig. 4). It has to be noted that 3-aminopropyl groups are present as alkylammonium cations in aqueous solutions. The electrostatic attraction forces have significant influence on the adsorption interactions of charged macromolecules of proteins with the surface of an electrically charged adsorbent [2, 26]. This interaction has been observed even earlier by our group [25, 27]. So-called hydrophobic interactions between the hydrophobic parts of the enzyme molecules and the alkyl groups located on the surface of functionalized magnetite have even some additional influence on the adsorption of urease. Moreover, increasing the length of the alkyl group permits to significantly enhance the adsorption of urease (compare samples 2 and 3 in Fig. 5). Note that this is in agreement with the calculated Langmuir constants: 8.6 for 1; 16.0 for 2; 13.1 for 3; and 0.2 for 4.
We have previously shown [28] that the adsorption of urease is less effective on non-functionalized magnetite samples (see Table 1). The adsorption of the enzyme increases substantially when magnetite is functionalized with amino groups. It is further increased with the introduction of hydrophobic alkyl groups into the functional layer, along with 3-aminopropyl groups (Table 1). The introduction of alkyl radicals has also a positive effect on the retention of adsorbed urease activity. As shown in Table 1, the retention of the enzyme activity is in this case is increased almost 2 times compared to non-functionalized magnetite.
Finally, we studied the changes in the activity of urease adsorbed on functionalized magnetite during storage of the samples. As we have shown previously [27], the polysiloxane matrices usually promote increased activity of immobilized urease on storage time. It was assumed that this was due to creation of the environment around the enzyme resembling the intracellular one. As it can be seen from Fig. 6, the residual urease activity for sample 1, containing only aminogroups in the surface layer, increases slightly during the first period of storage, and then decreases gradually. This can be explained by the stabilization of the structure of the enzyme on the early stages of the storage. The activity of immobilized enzyme decreases steadily during storage for samples 2 and 3 (Fig. 6). A different situation is observed in the case of a sample of 4—after the initial increase in the residual urease activity it is not reduced within 45 days (Fig. 6). Obvious factors that contribute to this effect are the increased length of the spacer and the presence of two active centers of adsorption.
4 Conclusions
Hydrolytic polycondensation reaction of trifunctional silanes (as functionalizing agents) and tetraethoxysilane (as structuring agent) allows the creation of mono- and bifunctional layers on the surface of Fe3O4 nanoparticles. The presence in such layers at the same time of amino and alkyl groups (methyl or n-propyl) increases the sorption capacity of the samples towards urease. Thus, increase in the length of alkyl radical causes a significant increase in adsorption. At the same time, introduction of the ethylenediamine group instead of amino group causes deterioration of the kinetic characteristics of the sample, and the sorption isotherm reaches a plateau at much higher concentrations of urease in the initial solution. However, in this sample, the adsorbed urease retains high activity for 45 days, while for the other samples it has been a subject of gradual decline. Finally, it should be noted that the obtained powder materials retain their magnetic properties, which allows them to be easily removed from the reaction mixture.
References
Eltekova NA, Eltekov AY (2000) Sieve effect at the adsorption of albumin and gamma globulin. Russ J Phys Chem 74:1075–1079 (in Russian)
Van der Veen M, Cohen Stuart M, Norde W (2007) Spreading of proteins and its effect on adsorption and desorption kinetics. Colloids Surf B Biointerfaces 54:136–142
Hofs B, Brzozowska A, de Keizer A et al (2008) Reduction of protein adsorption to a solid surface by a coating composed of polymeric micelles with a glass-like core. J Colloid Interface Sci 325:309–315
Tsapikouni ThS, Missirlis YF (2008) Protein-material interactions: from micro-to-nano scale. Mat Sci Eng B 152:2–7
Reichelt S, Eichhorn KJ, Aulich D et al (2009) Functionalization of solid surfaces with hyperbranched polyesters to control protein adsorption. Colloids Surf B Biointerfaces 69:169–177
Braun S, Rappoport S, Zusman R, Avnir D (1990) Ottolenghi M. Biochemically active sol–gel glasses. The trapping of enzymes. Mater Lett 10:1–8
Brinker CJ, Sheerer GW (1990) Sol–gel science. The physics and chemistry of sol–gel processing. Academic Press, San Diego
Kandimalla VB, Tripathi VS, Ju H (2006) Immobilization of biomolecules in sol–gels: biological and analytical applications. Crit Rev Anal Chem 36:73–106
Iler RK (1979) The chemistry of silica: solubility, polymerization, colloid and surface properties, and biochemistry. Wiley, New York
Avnir D, Coradin T, Lev O, Livage J (2006) Recent bio-applications of sol–gel materials. J Mater Chem 16:1013–1030
Coradin T, Boissiere M, Livage J (2006) Sol–gel chemistry in medicinal science. Curr Med Chem 13:99–108
Pogorilyi RP, Goncharyk VP, Yurchenko GR et al (2005) Immobilization of urease on functionalized polisyloxane xerogels and hydrogels obtained by sol–gel method. Quest Chem Chem Technol 6:103–106 (in Ukraininan)
Takahashi H, Li B, Sasaki T, Miyazaki C, Kajino T, Inagaki S (2001) Immobilized enzymes in ordered mesoporous silica materials and improvement of their stability and catalytic activity in an organic solvent. Microporous Mesoporous Mater 44–45:755–762
Pimentel MCB, Leao ABF, Melo EHM, Ledingham WM, Lima-Filho JL, Sivewright M, Kennedy JF (2007) Artificial cells. Blood Substit Biotechnol 35:221–235
Bayramoglu G, Arıca MY (2008) Preparation of poly(glycidylmethacrylate–methylmethacrylate) magnetic beads: application in lipase immobilization. J Mol Catal B Enzym 55:76–83
Ma Z, Guan Y, Liu H (2006) Superparamagnetic silica nanoparticles with immobilized metal affinity ligands for protein adsorption. J Magn Magn Mat 301:469–477
Melnik IV, Zub YuL, Alonso B, Abramov NV, Gorbik PP (2012) Creating of a functional polysiloxane layer on the surface of magnetic nanoparticles using sol–gel method. Glass Phys Chem 38:96–104
Melnyk IV, Zub YL (2012) Preparation and characterisation of magnetic nanoparticles with bifunctional surface layer ≡ Si(CH2)3NH2/≡ SiCH3 (or ≡ SiC3H7–n). Microporous Mesoporous Mater 154:196–199
Brunauer JS, Emmet PH, Teller E (1938) Adsorption of gases in multimolecular layers. J Am Chem Soc 60:309–319
Lurie YY (1984) Analytical chemistry of industrial wastewater. Chemistry, Moscow (in Russian)
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinium. J Am Chem Soc 40:1361–1403
Finn L, Slinyakova I (1975) The structure and thermal decomposition of the organopolysiloxane xerogels shown by IR spectroscopy. Colloid J 37:723–729 (in Russian)
Lin-Vien D, Colthup NB, Fateley WG, Grasselli JG (1991) The handbook of infrared and Raman characteristic frequencies of organic molecules. Academic Press, London
Giles CH, MacEwan TH, Nakhwa SN et al (1960) A system of classification of solution adsorption isotherms, and its use in diagnosis of adsorption mechanisms and in measurement of specific surface areas of solids. J Chem Soc 10:3973–3993
Bosker WTE, Iakovlev PA, Norde W, Cohen Stuart M (2005) BSA adsorption on bimodal PEO brushes. J Colloid Interface Sci 286:496–503
Pogorilyi RP, Goncharyk VP, Kozhara LI, Zub YL (2009) The influence on the activity of the adsorbed urease of structural and adsorption characteristics of polisyloxane matrices containing surface layer with 3-aminopropyl groups. Surface 1(16):35–45 (in Ukrainian)
Pogorilyi RP, Goncharyk VP, Kozhara LI, Zub YuL (2008) Covalent immobilization of urease on polysiloxane matrices containing 3-aminopropyl and 3-mercaptopropyl groups. Appl Biochem Microbiol 44:621–625 (in Russian)
Pogorilyi RP, Honcharyk VP, Melnyk IV, Zub YuL (2008) In: Innocenzi P, Zub Yu, Kessler V (eds) Sol–gel methods for materials processing, XII. Springer, Dordrecht
Acknowledgments
R. P. P., I. V. M. and Yu. L. Z. would like to thank the State Target Scientific and Technical Program “Nanotechnologies and Nanomaterials” (project 6.22.5.42) and TCPFR “Fundamental Problems of Nanostructural Systems, Nanomaterials, and Nanotechnologies” (project no. 57/12-H) of NAS of Ukraine. V. G. K. and G. A. S. express their gratitude to the Swedish Research Council for support of the project “Molecular Precursors and Molecular Models of Nanoporous Materials” and to the EU FP7 program for support of the EuRARE project.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Pogorilyi, R.P., Melnyk, I.V., Zub, Y.L. et al. Urease adsorption and activity on magnetite nanoparticles functionalized with monofunctional and bifunctional surface layers. J Sol-Gel Sci Technol 68, 447–454 (2013). https://doi.org/10.1007/s10971-013-2991-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-013-2991-z