Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Pharmaceuticals, as a large group of human and veterinary medicinal compounds, are considered as an emerging environmental problem, due to long term usage worldwide. Its continuous input and persistence to the aqueous system, even at low concentrations, may result in a potential risk for aquatic and terrestrial organisms. Cardiovascular drugs are among most frequently detected pharmaceuticals in wastewaters, where, according to literature, β-blocker metoprolol is listed in concentration of 10–390 ng L−1 [1]. Although in trace concentrations, those pharmaceuticals are persistent against biological degradation and they retain their chemical structure long enough and their presence in the environment is considered potentially dangerous.
Metoprolol tartrate salt (1-[4-(2 methoxyethyl)phenoxy]-3-(propan-2-ylamino)propan-2-ol tartrate (2 : 1), CAS No 56392-17-7, (C15H25NO3)2 C4H6O6, M r = 684.81, MET) is a selective β-blocker that is used to treat a variety of cardiovascular diseases, such as hypertension, coronary artery disease, and arrhythmias, by blocking the action of epinephrine and norepinephrine on the β-adrenergic receptors in the body, primarily in the heart [2]. Wide use of MET in recent years has a consequence in increasing concentrations of this pharmaceutical in aqueous effluents [3, 4]. MET shows slow direct phototransformation and/or hydrolysis [5, 6]. An efficient way to deal with this problem is degradation of the drug by advanced oxidation processes (AOPs) based on the formation of hydroxyl (•OH) and other radicals that oxidize toxic and non-biodegradable compounds to the different co-products, and, in the course of time, to inert final products [7]. MET contains a secondary amine group and a weakly/moderately activated aromatic ring that are probable targets of molecular ozone and attacks of •OH [2]. Yang et al. [8] investigated degradation of selected β-blockers (atenolol, metoprolol, and propranolol) in aqueous suspensions of TiO2 Degussa P25 and proposed a preliminary mechanism of degradation of these compounds. Romero et al. [9, 10] have also investigated and compared degradation intermediates of metoprolol and propranolol by AOPs.
Titanium dioxide (TiO2) is recognized as catalyst employed for almost all the pharmaceuticals photocatalytic treatment studies, as inexpensive, commercially available at various crystalline forms and particle characteristics, non-toxic and photochemically stable material [11]. Degussa P25, a commercially available powder is a mixture of anatase and rutile phases (with 80% of anatase approximately [12]), showing exceptional photocatalytic performance, superior to other grades of TiO2 [13].
We synthesized TiO2 nanopowders via sol–gel routine to obtain photocatalyst efficient in metoprolol degradation. The sol–gel is solution-based technique, where material structure is created through chemical reactions in liquid state. It has recently received more attention in production of high performance nanostructured materials, where morphology, surface area, average nanocrystallite size, crystallinity, and phase structure are controlled by sol–gel synthesis parameters [14]. The properties of sol–gel synthesized TiO2 nanopowders are determined by the key sol–gel processing parameters such as the precursor type, pH value in hydrolysis process, duration and temperature of hydrolysis (aging), type of alcogel, duration and temperature of drying, and calcination conditions (heating rate, temperature, duration and cooling rate) [15].
In this paper we investigate the influence of sol–gel synthesis condition on photocatalytic properties of TiO2 nanopowders, by varying some synthesis parameters (precursor, alcohol, basic solution, and calcination temperature) aiming to obtain photocatalyst for degradation of metoprolol, efficient enough in comparison to Degussa P25. Several methods of characterization, such as the X-ray diffraction (XRD), scanning electron microscopy (SEM), energy dispersive X-ray spectroscopy (EDS), Brunauer-Emmett-Teller (BET) measurements, Fourier transform infrared (FTIR) and Raman spectroscopy, are employed to correlate structural and morphological properties of synthesized TiO2 nanopowders and their photocatalytic activity under ultraviolet (UV) irradiation.
2 Experimental
2.1 Powder synthesis
The TiO2 nanopowders used as catalysts in this study are synthesized by the sol–gel method, which typically involves hydrolysis of a solution of a precursor molecule in order to obtain a suspension of colloidal particles—gel (at first in the form of hydrogel and then in the form of alcogel). Titanium tetrachloride (TiCl4) and tetrabutyl titanate (Ti(OC4H9)4) are the precursors commonly used in sol–gel synthesis, which required different procedures in alcogel production:
(1) In synthesis with tetrabutyl titanate as precursor, ethanol (EtOH) is used as the solvent, hydrochloride acid as the catalyst, and water for hydrolysis. The hydrolysis and polycondensation reactions of Ti(OC4H9)4 were carried out at the room temperature, whereas the measured pH value of solution was 7.0. The reagent molar ratio was Ti(OC4H9)4:EtOH:HCl:H2O = 1:15:0.3:4 [16]. After the gelation, the wet gels were dried at 80 °C;
(2) In synthesis with titanium tetrachloride as the precursor, Ti(OH)4 hydrogel was obtained by hydrolysis of TiCl4 at 0 °C with controlled addition of 2.5 wt% alkalic solution (NH4OH or NaOH) into the aqueous solution of TiCl4 (0.3 M) and careful control of the pH value (9.3) of the solution [15]. After aging (fixed time of 5 h) [17], as-prepared hydrogel was filtered and rinsed out with distilled water until complete removal of chloride ions. The absence of Cl– ions was checked using 0.1 M solution of AgNO3. Obtained Ti(OH)4 hydrogel was converted to its alcogel by repeated exchange with corresponding alcohol for several times. Also, different alcohols were used to investigate the influence of the type of alcogel on properties of photocatalysis: methanol, ethanol, 2-propanol, amylalcohol and glycerol. The alcogels were dried at 280 °C during 4 h.
The TiO2 nanopowders produced by one of the procedures described above are amorphous. During the calcination process at temperature of 350 °C or higher, the crystalline anatase phase is formed [18]. As calcination temperature approaches ~600 °C, the phase transition from anatase to rutile starts [19]. However, high calcination temperature results in the growth of nanocrystalline particles and the rapid decrease of specific surface area. To investigate the influence of temperature of calcination, few samples synthesized with the same alcohol (amylalcohol) were exposed to different calcination temperatures (400, 550 and 650 °C). The duration of calcination process (7 h) and temperature gradient of the heating (135 °C h−1) were the same in all the experiments.
The scheme of the TiO2 production by sol–gel process can be written as follows:
The samples synthesized by described procedures are listed in Table 1, together with variable parameters of synthesis (precursor, alcohol, basic solution, and calcination temperature).
All chemicals in this experiment were analytical grades, used as received. Tetrabutyl titanate 99% was produced by Acros Organics, TiCl4 and glycerol by Merck Chemicals, amylalcohol by Lacheme, ethanol by J. T. Backer, methanol and 2-propanol by Centrohem, 25% NH4OH by Carbo Erba and NaOH by MP Hemija.
2.2 Characterization methods
Powder XRD patterns of TiO2 nanopowders were recorded by Siemens X-ray Diffractometer (Kristaloflex 500) with Ni filtered CuKα radiation and using Si as an external standard. The measurements were performed in the 2θ range from 20° to 80° in a continuous scan mode with a step width of 0.02° and at a scan rate of 1° 2θ min−1.
Morphology of the catalyst is determined by SEM. Gold coated particles were observed on SEM type JEOL JSM-6460LV with the operating voltage of 20 keV. Composition/quality of TiO2 was analyzed on SEM with EDS detector. EDS are performed by INCAx-sight detector and “INAx-stream” pulse processor (Oxford Instruments).
The powder specific surface area of the samples was calculated following the multipoint BET procedure on Quantachrome ChemBet-3000 setup. The particle size distribution (PSD) was derived from nitrogen adsorption/desorption isotherms obtained at 77 K.
The IR reflection spectra of TiO2 nanopowders pressed into pellets were measured at room temperature using a BOMEM-DA8 spectrometer in the spectral range between 400 and 4,000 cm−1.
Raman scattering measurements of TiO2 samples was performed in the backscattering geometry at room temperature in the air using Jobin–Yvon T64000 triple spectrometer, equipped with a confocal microscope and a nitrogen-cooled charge coupled device detector. The spectra excited by 514.5 nm line of Ar+/Kr+ ion laser with output power less than 5 mW to avoid local heating due to laser irradiation.
2.3 Photocatalytic activity
The photocatalytic activity of the TiO2 nanopowders was evaluated by the degradation of the solution of metoprolol tartrate salt (≥99%, Sigma–Aldrich). Photocatalytic degradation was carried out in a cell made of Pyrex glass (total volume of ca. 40 mL, liquid layer thickness 35 mm), with a plain window on which the light beam was focused. The cell was equipped with a magnetic stirring bar and a water circulating jacket. A 125 W high-pressure mercury lamp (Philips, HPL-N, emission bands in the UV region at 304, 314, 335 and 366 nm, with maximum emission at 366 nm), together with an appropriate concave mirror, was used as the radiation source. The output for the mercury lamp was calculated to be ca. 8.8 × 10−9 Einstein mL−1 min−1 (potassium ferrioxalate actinometry).
In a typical experiment the initial MET concentration was 0.05 mM and the TiO2 loading was 1.0 mg mL−1, unless otherwise is stated. The total suspension volume was 20 mL. The aqueous suspension of TiO2 was sonicated (50 Hz) in the dark for 15 min before illumination, to uniformly disperse the photocatalyst particles and attain adsorption equilibrium. The suspension thus obtained was thermostated at 25 ± 0.5 °C in a stream of O2 (3.0 mL min−1), and then irradiated. During irradiation, the mixture was stirred at a constant rate under continuous O2 flow. Control experiments were carried out under O2 flow but by stopping the irradiation showed that there were no losses of volatile compounds during the degradation. All experiments were performed at the natural pH.
For the LC–DAD kinetic studies of MET photodegradation, aliquots of 0.50 mL were taken from the reaction mixture at the beginning of the experiment and at regular time intervals. Aliquot sampling caused a maximum volume variation of ca. 10% in the reaction mixture. The suspensions containing photocatalyst were filtered through Millipore (Milex-GV, 0.22 μm) membrane filter. Lack of adsorption of MET on the filters was preliminary checked. After that, a 20-μL sample was injected and analyzed on an Agilent Technologies 1100 Series liquid chromatograph, equipped with an Eclypse XDB-C18 column (150 mm × 4.6 mm i.d., particle size 5 μm, 25 °C). The UV/vis DAD detector was set at 225 nm (wavelength of MET maximum absorption). The mobile phase (flow rate 0.8 mL min−1) was a mixture of acetonitrile (ACN, 99.8%, J. T. Baker) and water (with the following gradient: 0 min 15% ACN, 5 min 30% ACN and 5 min 30% ACN; post time 3 min), the water being acidified with 0.1% H3PO4 (85%, Lachema, Neratovice).
Commercially available TiO2, Degussa P25 (75% anatase and 25% rutile, specific area of 50 m2 g−1, and average particle size about 20 nm, according to the producer’s specification), was used for the purpose of comparison.
3 Results
3.1 XRD
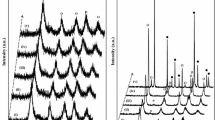
The XRD patterns of chosen TiO2 nanopowders are presented in Fig. 1. The diffraction peaks present in all samples coincide to anatase TiO2 structure (JCPDS card 21-1272, space group I41/amd). Their positions are slightly shifted, indicating a change in lattice parameters in comparison to the bulk values for anatase (a 0 = 0.37852 nm, c 0 = 0.95139 nm), as shown in Table 2. Consequently, the lattice volume of anatase in all synthesized nanopowders is smaller than in its bulk counterpart (V 0 = 136.3127 × 10−3 nm). It has been noticed that positive pressure caused by large surface hydration could be a primary reason for lattice contraction volume in anatase nanostructures [20, 21].
The average crystallite size of the anatase nanoparticles, estimated by using Scherrer’s equation, is in range from 13 to 17 nm, as listed in Table 2. A whole powder pattern structure refinement using the structure analysis software Power Cell [22] was also carried out. As expected, structural analysis gives slightly higher values for crystallite size in comparison to Scherrer method, because the effects of microstrain are taken into account.
Note that diffraction peaks of brookite phase are not seen in XRD patterns of TiO2 nanopowders, whereas the peaks corresponding to the rutile phase (JCPDS card no. 21-1276, space group P42/mnm) are observed in the diffractogram of sample T(CAN)650. The amount of rutile phase in this sample is estimated to be ~30% according to Power Cell. The lack of the reliable results for crystallite size and microstrain in T(CAN)650 is probably related to the presence of rutile phase in this sample.
3.2 SEM/EDS
The SEM images of two synthesized TiO2 nanopowders (T(CAA)550, T(CAN)650) and Degussa P25 are presented in Fig. 2. As can be seen from the micrographs, most of the particles in this sample have a spherical shape, with a grain diameter, roughly estimated to be about 23 nm in synthesized samples, and around 29 nm in Degussa P25. Table 3 presents the chemical composition of the same nanopowders, estimated by EDS spectra (Fig. 3, upper row), which correspond to framed area of micrographs (Fig. 3, lower row). In the samples analyzed here, oxygen weight percent is higher than in stoichiometric TiO2 (40 wt%), with the exception of the sample T(CAN)550. Among synthesized samples, the highest value of oxygen weight percent (but still lower than value in Degussa P25) is registered in T(CAN)650. The EDS analysis has also shown the presence of sodium (Na) and very low concentration (<1 wt%) of chlorine (Cl) in the samples synthesized from NaOH as basic solution and TiCl4 as precursor (Table 3). Note here also that hydrogen cannot be detected by EDS method.
3.3 BET
The values of the BET specific surface areas (S BET ) of some TiO2 nanopowders are given in the Table 4, together with pore volume (V p ) and pore diameter (D p ). The sample T(CAN)400 exhibits the lowest specific surface area (32 m2 g−1), whereas S BET ranges between 51 and 63 m2 g−1 in the rest of the samples. All nanopowders listed here, as well Degussa P25, are mostly mesoporous (pore diameters of 2–50 nm, S BET ≈ S meso ). However, mean pore diameter of synthesized samples, which is in the range of 6–7 nm, is much lower than the corresponding one of Degussa P25 (31.5 nm [12]).
The PSD of specified synthesized samples are bimodal (Fig. 4), with the first mode peaked in the range 2–3 nm, and the second mode around 4 nm. Among these samples, the T(CAN)650 contains the greatest number of pores with diameter higher than 3 nm, whereas the number of such pores in T(CAN)400 is the smallest.
3.4 FTIR
The IR reflectivity spectra of representative TiO2 nanopowders in the range of 450–4,000 cm−1 are presented in Fig. 5. The spectra are dominated by the features in the range of 450–1,000 cm−1, which can be related to the surface vibration of Ti–O bonds [23, 24]. It is obvious that more features in this region appear in the samples with mixed anatase/rutile phase (T(CAN)650 and Degussa P25), as rutile has one more IR active mode than anatase in specified region [25]. Also, nanostructure of analyzed powders has strong influence on the shape of IR reflectivity spectra [26], but this is beyond the scope of this study.
The reflectivity spectra of the same samples in the region of 1,200–4,000 cm−1 are shown enlarged in the Inset of the Fig. 5. The main spectral features in this region appear around 1,260 cm−1, between 1,400 and 1,800 cm−1, and in the ranges of 2,800–3,000 cm−1 and 3,200–3,800 cm−1. The band at about 1,260 cm−1, which is most prominent in the IR spectrum of Degussa P25, was assigned to carbonate [27] or nitrate [28] species at TiO2 surface. Small bands in the range of 1,400–1,800 cm−1 most probably originate from carbonates or hydrogen carbonates groups in TiO2 [29]. The bands in the range of 2,800–3,000 cm−1 can be assigned to the symmetric and asymmetric stretching mode of the C–H bond [30], originating from residues which remain in TiO2 samples even after calcination.
As TiO2 surface O–H group nature and distribution are in many cases determining factor of catalyst behaviour, a considerable research has been devoted here to characterizing these groups present at the surface of TiO2. According to many FTIR studies published in the literature, the vibration band between 3,000 and 3,150 cm−1 is associated with the O–H stretching mode of water molecules [31], while that at about 1,630 cm−1 is assigned to O–H bending modes [23 and references therein]. The bands in frequency range of 3,150–3,500 cm−1 are usually attributed to H-bounded O–H groups, and those in the 3,400–3,600 cm−1 region to mixed O–H groups [31]. The bands in the 3,600–3,800 cm−1 region are due to stretching modes of different types of free hydroxyl groups adsorbed on the crystalline surfaces of titania or on defects located at the surfaces [23 and references therein]. It should be noted that IR reflectivity spectra of TiO2 nanopowders, shown in Fig. 5, enable identification of specified surface groups, but do not allow determination of the relation between the amounts of different kinds of these groups at the surface of TiO2 nanoparticles.
3.5 Raman scattering
The Raman spectra of the samples synthesized by sol–gel method, listed in Table 1, were the subjects of analysis, which indicated anatase as a dominant phase in all samples. The spectrum of the sample T(CAA)550 shown in Fig. 6a illustrates characteristic anatase Raman modes in the region of 50–1,000 cm−1 and so called C–H and O–H spectral region in the 1,000–4,000 cm−1 frequency range.
a Raman spectrum of T(CAA)550 nanopowder. Spectra of TiO2 nanopowders divided in three characteristic regions: b around anatase E g mode, c 210–380 cm−1 (brookite modes), and d 350–700 cm−1 (anatase, rutile and sodium titanate modes). The intensities of the spectra are normalized to 1 in the first region (E g ) and multiplied by 50 and 7 in following two regions to pronounce corresponding features of the spectra. Anatase, rutile, brookite and sodium titanate are labelled as A, R, B, and ST, respectively
Generally, the intensity and linewidth of Raman modes provide the information about crystalline quality of the material. According to this, among synthesized samples, T(CAN)650 and T(BE)550, with the most intensive and narrowest characteristic anatase modes in Raman spectra, have the crystalline quality close to Degussa P25.
In order to point out some features related to different TiO2 phases (anatase, brookite and rutile) and the presence of residuals due to synthesis process, Raman spectra of some nanopowders (T(CAA)550, T(CAN)400, T(CAN)550, and T(CAN)650), together with Degussa P25, are shown in Fig. 6b–6d. The spectra are divided in three characteristic regions because of different intensities in each of them.
The first region, around anatase E g Raman mode (~143 cm−1), is shown in Fig. 6b. The relationship between Raman shift and full width at half maximum (FWHM) of this mode, for all TiO2 nanopowders, is presented in Fig. 7. These results show that the Raman shift and FWHM of sol–gel synthesized samples are greater than in Degussa P25 nanopowder: Raman shift is positioned between 142.6 cm−1 in T(CAN)650 and 144.1 cm−1 in T(CGA)550, whereas the variation of FWHM ranges from 9.4 cm−1 in T(BE)550 to 12.6 cm−1 in T(CPA)550. Beside the crystalline quality, Raman shift and linewidth of E g mode in anatase nanocrystals at given temperature also depend on confinement effects, due to the nanocrystalline size, microstrain and nonstoichiometry [32, 33]. In order to estimate which of those effects has the greatest influence on blueshift and broadening of Raman E g mode of synthesized nanopowders in comparison to corresponding values in bulk anatase (141 cm−1 and 8 cm−1 [34]), the relationship between Raman shift and FWHM is calculated by phonon confinement model (PCM) (Fig. 7). The particle size used in the calculation belongs to the range of 17–25 nm, corresponding to the size of nanocrystallites in synthesized powders, estimated from XRD (Table 2). However, for the specified crystallite sizes, the values of Raman shift and FWHM calculated by PCM are lower than experimental ones, even when PCM with inhomogeneous strain is applied (open circles in Fig. 7) [35, 36]. Although widening of E g mode can be the consequence of lower crystalline quality, it is obvious that, in addition to phonon confinement and microstrain, the nonstoichiometry must be included in the explanation of blueshift and broadening of Raman E g mode in most of our samples. Namely, it is well known that this mode is blueshifted and broadened due to nonstoichiometry in anatase nanopowders [37].
The relationship between Raman shift and FWHM of E g mode of anatase phase in TiO2 nanopowders. The experimental values are denoted by stars (the calcination temperature is intentionally omitted in the names of all samples calcined at 550 °C for the clarity). Open symbols denote the values calculated by PCM without strain (triangles), and including strain effect (circles)
In order to investigate the presence of brookite phase by Raman spectroscopy, the second region of the Raman spectra, from 210 to 380 cm−1 is analyzed. This part of the spectra is shown in Fig. 6c for previously chosen set of samples. The brookite modes are located as follows: A 1g (~247 cm−1), B 3g (~288 cm−1), B 1g (~322 cm−1), and B 2g (~366 cm−1) [38]. Low intensities and large widths of these modes indicate great disorder and partial amorphization of brookite in all the samples. To estimate the amount of brookite phase, the sum of the integrated intensities of Lorentzian peaks, originating from the brookite modes (ΣI B ), was compared to the intensity of Lorentzian peak related to the B 1g mode of anatase phase (I A (B 1g )). Although the estimation of brookite modes intensities is unreliable to some extent, due to mode overlapping, a variation in brookite content with synthesis conditions may be roughly estimated from the intensity ratio of brookite to anatase modes, ΣI B /I A (B 1g ), shown in Fig. 8. In such a way, the amount of brookite phase in the samples synthesized with NH4OH basic solution is higher than in the samples synthesized with NaOH basic solution. Note also that additional mode at about ~275 cm−1, which can be related to the sodium titanate [39], is registered in the Raman spectra of the samples synthesized from NaOH basic solution.
The ratio of the total brookite modes intensity, ΣI B , and the anatase B 1g Raman mode intensity I A (B 1g ) in TiO2 nanopowders. The inset: Lorentzians of brookite modes in the experimental Raman spectra of sample T(CPA)550. (The calcination temperature is omitted in the names of the samples calcined at 550 °C for clarity)
The Raman spectra in the region from 350 to 700 cm−1 (Fig. 6d) of all TiO2 nanopowders are dominated by anatase modes B 1g (~399 cm−1), A 1g + B 1g (~518 cm−1), and E g (~639 cm−1) [40]. However, the modes of rutile phase, E g (~447 cm−1) and A 1g (~612 cm−1) [40], appear in this region in the spectra of samples T(BE)550 (synthesized with tetrabutyl titanate as a precursor) and T(CAN)650 (calcined at 650 °C), as well as in Degussa P25.
Compared with IR spectroscopy [41], Raman spectroscopy has rarely been applied to study the surface complex formation at anatase nanoparticles [42]. Namely, in C–H and O–H spectral region, Raman features are hard to resolve as sample luminescence is often too intensive. Nevertheless, we demonstrate here the applicability of Raman spectroscopy to identify specified surface groups and estimate their amount at the surface of TiO2 nanoparticles.
Raman spectra of representative TiO2 nanopowders in the region between 1,000 and 4,000 cm−1 are shown in Fig. 9. This region is dominated by the broad feature between 2,800 and 3,800 cm−1 and less intensive mode at about 1,650 cm−1. According to many vibrational studies, carried out to determine the characteristics of water molecules and hydroxyl groups adsorbed on anatase surfaces [29 and references therein], broad Raman structure in the frequency range of 2,800–3,800 cm−1 can be assigned to O–H stretching vibration of water molecules adsorbed on the surface of the particles and condensed in the pores of TiO2 nanopowders [42]. The intensity of this structure is the highest in the Raman spectra of Degussa P25 and T(CAA)550, followed by T(CAN)400, implying that the amount of adsorbed water molecules is the greatest in those samples. Also, the feature peaked at the frequency between 1,625 and 1,670 cm−1, which can been assigned to the bending modes of adsorbed water [29], is the most intensive in Degussa P25 and T(CAA)550. Complex shape and wideness of the spectral features described above point out to the variety of components in this range, indicating the presence of several types of surface sites bearing hydroxyl groups and adsorbed H2O molecules [43, 44].
In addition, several sharp peaks are noticed in the Raman spectra shown in Fig. 9. A sharp peak at ~1,055 cm−1, registered in the spectra of T(CAN)400, T(CAN)550 and T(CAN)650, most probably belongs to stretching vibration of NO3 −, due to the presence of NaNO3 [45], as a consequence of using NaOH basic solution in the synthesis process. The peaks in the frequency range 1,200–1,500 cm−1, most prominent in Degussa P25 and T(CAA)550, can be associated with carbonate groups [23], whereas the peak at 2,940 cm−1 can be assigned to the stretching mode characteristic for CH- species [46].
However, low intensity peak located at ~3,700 cm−1 noticeable in all the samples except T(CAN)400, is the most interesting one in our analysis. Namely, this feature, which is assigned to the O–H stretching mode of isolated (free) hydroxyls [29], can be related to the ability of the anatase samples to form the surface O–H groups with the important influence on their catalytic activity [47]. According to several authors, the hydroxyl groups with vibration frequency at about 3,700 cm−1 are the most photocatalytically active among all hydroxyl groups at the surface of TiO2 nanoparticles [23].
The analysis of Raman data presented above has demonstrated the ability of Raman spectroscopy not only to characterize TiO2 in terms of structure and crystallite size, the presence of different crystal modifications, microstrain and nonstoichiometry, but also to recognize surface O–H groups in TiO2 nanopowders, important for photocatalytic activity.
3.6 Photocatalysis
The influence of the different synthesis parameters on the photocatalytic activity of TiO2 nanopowders was studied on the example of the photocatalytic degradation of MET under UV light irradiation. The results are presented in Fig. 10, together with the results obtained using commercially available Degussa P25.
The Fig. 10a illustrates the influence of the type of precursor on TiO2 nanopowders photocatalytic activity in the photodegradation of MET. As can be seen, the catalytic activity of TiO2 nanopowders synthesized with titanium tetrachloride as the precursor is much higher, but still somewhat lower than of Degussa P25.
The influence of the type of alcogel on photocatalytic activity of TiO2 nanopowders (Fig. 10b) was examined using different alcohols. As can be seen, the highest photocatalytic activity showed the catalysts obtained using methanol and amylalcohol.
The Fig. 10c shows the influence of calcination temperature on the photocatalytic activity of TiO2 nanopowders on the MET photodegradation. It can be seen that the TiO2 nanopowders calcined at ≥550 °C showed higher photocatalytic activity, even slightly higher than Degussa P25.
Since T(CAN)650 showed the highest photocatalytic activity in the photodegradation of MET, the effect of TiO2 loading on the efficiency of MET photodegradation was examined in the loading range from 0.5 to 5.0 mg mL−1 (Fig. 11a). As can be seen, with the increase of the TiO2 loading up to 2 mg mL−1, the degradation rate increases, and afterwards decreases. Theoretically, the increase in the catalyst loading above an optimum value has no effect on the photodegradation rate since all available light is already utilized. However, higher loading of TiO2 led to the aggregation of its particles and thus to a decrease in the contact surface between the reactant and photocatalyst particles, which caused a decrease in the number of active sites, resulting in a lower rate of photodegradation. Also, when TiO2 was overdosed, the intensity of the incident UV light was attenuated because of the decreased light penetration and increased scattering, which attenuated the positive effect coming from the dosage increment, and therefore the overall performance decreased [48].
A similar effect was also observed with Degussa P25 (Fig. 11b), but the optimal TiO2 loading was 1 mg mL−1. It should be pointed out that, T(CAN)650 showed higher photocatalytic activity in the whole range of the catalyst loading.
4 Discussion
On the basis of experimental results presented in previous sections, we shall analyze the dependence of TiO2 nanopowder properties on sol–gel synthesis conditions, and the impact of their structural, morphological and surface properties on photocatalytic degradation of metoprolol under UV irradiation.
Structural analysis of synthesized nanopowders, performed by XRD and Raman spectroscopy, has shown that the sample T(CAN)650, with the majority of structural characteristics similar to Degussa P25, has the highest photocatalytic activity. Namely, the mixture of anatase and rutile (with negligible amount of brookite) in this sample is similar to Degussa P25. At the same time, high intensity of Raman signal of T(CAN)650, with the position and linewidth of E g anatase mode (the closest to Degussa P25 among all synthesized powders) points to high crystalline quality of anatase of this sample. However, notice that T(BE)550 also has intensive Raman signal, with narrow E g anatase mode, but its catalytical activity is relatively poor compared to T(CAN)650. Despite that, T(CAN)550 has lower Raman intensity, with considerably greater blueshift and linewidth of E g mode, but its photocatalytic activity is close to T(CAN)650. From this point of view we may conclude that structural properties of nanopowders have some impact on photocatalytic activity, but not crucial.
On the other side, the results obtained from BET analysis for specific surface area, and especially pore size distribution, lead to unambiguous conclusions. Namely, larger surface area and greater number of pores with dimensions in mesoporus region (>2 nm), produce higher photocalytical degradation of metoprolol, as a material with relatively large molecules (1.7 nm in length). Note that higher mesoporosity is obtained in the samples synthesized in the presence of amyl-alcohol and is getting higher with increase of calcination temperature up to 650 °C.
Special attention must be also paid to analyze the amount of surface O–H groups in TiO2 nanopowders. Firstly, the lattice contraction, obtained by XRD analysis, points to increased hydration in all synthesized nanopowders. Also, the results for chemical composition obtained from EDS analysis, show higher oxygen content than in stoichiometric TiO2 for majority of the samples, except the sample T(CAN)550, which is obviously nonstoichiometric. However, the EDS results cannot give information about the form of the excess oxygen in TiO2 (which can be in the form of interstitial oxygen in the lattice, molecular oxygen at the surface or oxygen participating in OH groups). More information on the character and amount of O–H groups in our powders we obtained from IR, and especially from Raman spectra in the C–H and O–H spectral region. These results show that the frequencies and character of surface hydroxyl modes strongly depend on the morphology of the nanoparticles, sol–gel routes and type of TiO2 crystallites (anatase or rutile) formed during thermal treatment.
According to the presented results, the content of surface O–H groups, mostly related to adsorbed water molecules, is the highest in T(CAA)550, followed by T(CAN)400. However, in terms of photocatalytic activity, the presence of isolated hydroxyl groups on the surface of TiO2 is more important than the amount of adsorbed water molecules and surface hydroxyl groups [49]. Namely, it has been recently demonstrated that surface hydroxyl groups, which can trap holes to generate the photocatalytically important active species, i.e. •OH radicals, represent decisive factor in high photocatalytic activity of TiO2 [49]. Reactive hydroxyl radicals •OH are the precursors of degradation of any organic and inorganic compound and the first step in the photocatalytic degradation is the formation of the hydroxyl radicals from the TiO2 surface [50]. Indeed, the Raman results presented in Fig. 9 show that, despite the highest intensity of Raman modes associated to O–H groups related to adsorbed water in samples T(CAA)550 and T(CAN)400, these samples exhibit worse photocatalytic activity than T(CAN)550 and T(CAN)650. Note that the presence of high water content in T(CAN)400 is surely related to relatively low calcination temperature (400 °C). Moreover, the appearance of mode at about 3,700 cm−1, originating from isolated O–H groups, in the Raman spectra of all samples with relatively high photocatalytic activity (including Degussa P25), and the absence of this mode in T(CAN)400 with relatively poor activity, confirm the conclusions proposed above [49, 50].
5 Conclusion
We have demonstrated that highly photoactive nanocrystalline TiO2 powders can be produced by appropriate choice of sol–gel synthesis parameters. Different mixtures of TiO2 crystal modifications (anatase, rutile, and brookite) are obtained with proper choice of synthesis parameters: the type of precursor, alcohol and basic solution, as well as the calcination temperature. It is noticed that the content of brookite decreases photocatalytic activity in synthesized nanopowders with other properties comparable. On the other side, the amount of rutile does not have critical impact on the photocatalytic activity. The photocatalytic performances have been attributed to the anatase particle size and crystalline quality, rather than to the presence of rutile phase. Namely, the photocatalytic activity of TiO2 nanopowders mostly depends on anatase structure (crystallinity, particle size, microstrain, and nonstoichiometry) and porosity, as well as the amount of surface O–H groups, which can produce hydroxyl radicals. Additionally, pore size distribution in TiO2 nanopowders has crucial role in photocatalytic degradation of metoprolol, as a material with relatively large molecules. According to this, the highest photocatalytic degradation of MET is obtained for the sample synthesized by sol–gel routine with TiCl4 as a precursor, in the presence of amyl-alcohol and NaOH as basic solution, and calcined at 650 °C.
References
Klavarioti M, Mantzavinos D, Kassinos D (2009) Environ Int 35:402–417
Ikehata K, Naghashkar NJ, El-Din MG (2006) Ozone-Sci Eng 28:353–414
Alder AC, Schaffner C, Majewsky M, Klasmeier J, Fenner K (2010) Water Res 44:936–948
Rivas FJ, Gimeno O, Borralho T, Carbajo M (2010) J Hazard Mater 179:357–362
Liu QT, Cumming RI, Sharpe AD (2009) Photochem Photobiol Sci 8:768–777
Piram A, Salvador A, Verne C, Herbreteau B, Faure R (2008) Chemosphere 73:1265–1271
Dalrymple OK, Yeh DH, Trotz MA (2007) J Chem Technol 82:121–134
Yang H, An T, Li G, Song W, Cooper WJ, Luo H, Guo X (2010) J Hazard Mater 179:834–839
Romero V, De la Cruz N, Dantas RF, Marco P, Giménez J, Esplugas S (2011) Catal Today 161:115–120
Abramović B, Kler S, Šojić D, Laušević M, Radović T, Vione D (in press) J Hazard Mater
Doll TE, Frimmel FH (2003) Chemosphere 52:1757–1769
Colón G, Hidalgo MC, Navío JA (2001) J Photoch Photobiol A 138:79–85
Hurum DC, Agrios AG, Gray KA, Rajh T, Thurnauer MC (2003) J Phys Chem B 107:4545–4549
Baiju KV, Shukla S, Sandhya KS, James J, Warrier KGK (2007) J Phys Chem C 111:7612–7622
Golubović A, Šćepanović M, Kremenović A, Aškrabić A, Berec V, Dohčević-Mitrović Z, Popović ZV (2009) J Sol–Gel Sci Techn 49:311–319
Du YL, Deng Y, Zhang MS (2006) J Phys Chem Solids 67:2405–2408
Bala H, Guo Y, Zhao X, Zhao J, Fu W, Ding X, Jiang Y, Yu K, Lv X, Wang Y (2006) Mater Lett 60:494–498
Liu AR, Wang SM, Zhao YR, Zheng Z (2006) Mater Chem Phys 99:131–134
Sugimoto T, Zhou X, Muramatsu A (2003) J Colloid Interf Sci 259:43–52
Swamy V, Menzies D, Muddle BC, Kuznetsov A, Dubrovinsky LS, Dai Q, Dmitriev V (2006) Appl Phys Lett 88(1–3):243103
Li G, Li L, Boerio-Goates J, Woodfield BF (2005) J Am Chem Soc 127:8659–8666
Kraus W, Nozle G (2000) Power cell program for windows. BAM, Berlin
Begin-Colin S, Gadalla A, Le Caer G, Humbert O, Thomas F, Barres O, Villieras F, Toma LF, Bertrand G, Zahraa O, Gallart M, Honerlage B, Gilliot P (2009) J Phys Chem C 113:16589–16602
Gonzalez RJ, Zallen R, Berger H (1997) Phys Rev B 55:7014–7017
Gervais F, Baumard JF (1977) Solid State Commun 21:861–865
Grujić-Brojčin M, Šćepanović MJ, Dohčević-Mitrović ZD, Hinić I, Matović B, Stanišić G, Popović ZV (2005) J Phys D Appl Phys 38:1415–1420
Franking RA, Landis EC, Hamers RJ (2009) Langmuir 25:10676–10684
Li L, Shen Q, Cheng J, Hao Z (2010) Catal Today 158:361–369
Soria J, Sanz J, Sobrados I, Coronado JM, Maira AJ, Hernandez-Alonso MD, Fresno F (2007) J Phys Chem C 111:10590–10596
Goncalves RH, Schreiner WH, Leite ER (2010) Langmuir 26:11657–11662
Araña J, Doña-Rodrıguez JM, González-Díaz O, Tello Rendón E, Herrera Melián JA, Colón G, Navío JA, Pérez Peña J (2004) J Mol Catal A-Chem 215:153–160
Šćepanović MJ, Grujić-Brojčin MU, Dohčević-Mitrović ZD, Popović ZV (2006) Mat Sci Forum 518:101–106
Šćepanović MJ, Grujić-Brojčin MU, Dohčević-Mitrović ZD, Popović ZV (2007) Appl Phys A 86:365–371
Wang X, Shen J, Pan Q (2011) J Raman Spec 42:1578–1582
Spanier JE, Robinson RD, Zhang F, Chan S-W, Herman IP (2001) Phys Rev B 64(1–8):245407
Grujić-Brojčin MU, Šćepanović MJ, Dohčević-Mitrović ZD, Popović ZV (2009) Acta Phys Polonica A 116:5–54
Parker JC, Siegel RW (1990) Appl Phys Lett 57:943–945
Tompsett GA, Bowmaker GA, Cooney RP, Metson JB, Rodgers KA, Seakins JM (1995) J Raman Spectrosc 26:57–62
Liu H, Yang D, Zheng Z, Ke X, Waclawik E, Zhu H, Frost RL (2010) J Raman Spectrosc 41:1331–1337
Zhang Y-H, Chan CK, Porter JF, Guo W (1998) J Mater Res 13:2602–2609
Deiana C, Fois E, Coluccia S, Martra G (2010) J Phys Chem C 114:21531–21538
Watson S, Beydoun D, Scott J, Amal R (2004) J Nanoparticle Res 6:193–207
Cerrato G, Magnacca G, Mortera C, Montero J, Anderson JA (2009) J Phys Chem C 113:20401–20410
Martra G (2000) Appl Catal A: General 200:275–285
Asher SA, Tuschel DD, Vargson TA, Wang L, Geib SJ (2011) J Phys Chem A 115:4279–4287
Brownson JRS, Tejedor–Tejedor MI, Andersonbrownson MA (2005) Chem Mater 17:6304–6310
Yang D, Liu H, Zheng Z, Yuan Y, Zhao J, Waclawik ER, Ke X, Zhu H (2009) J Am Chem Soc 131:17885–17893
Abramović B, Šojić D, Despotović V, Vione D, Pazzi M, Csanádi J (2011) Appl Catal B Environ 105:191–198
Liu G, Sun C, Cheng L, Jin Y, Lu H, Wang L, Smith SC, Lu GQ, Cheng H-M (2009) J Phys Chem C 113:12317–12324
Vinu R, Madras G (2010) J Indian I Sci 90:189–230
Acknowledgments
This work was financially supported by the Serbian Ministry of Education and Science Projects No. III45018, ON171032, and ON172042, as well as SASA project F-134, and the Swiss National Science Foundation through Grant No. IZ73Z0-128169.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Šćepanović, M., Abramović, B., Golubović, A. et al. Photocatalytic degradation of metoprolol in water suspension of TiO2 nanopowders prepared using sol–gel route. J Sol-Gel Sci Technol 61, 390–402 (2012). https://doi.org/10.1007/s10971-011-2639-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-011-2639-9