Abstract
The rare-earth orthoferrites RFeO3 (R, rare-earth element) crystallize in an orthorhombic distorted perovskite structure. RFeO3 compounds exhibit interesting physical and chemical properties because of their ionic and electronic defects. Polycrystalline nano-sized RFeO3 powders were synthesized by the sol–gel combustion method. X-ray powder diffraction indicated that nanocrystalline powders were single ReFeO3 phase, which are agglomerated with average crystallite size of 60–90 nm estimated with the Scherrer’s equation. Magnetic measurements were carried out using a superconducting quantum interference device magnetometer. The influence on hysteresis curve of electronic structure of rare-earth element was investigated for R = Y, La and Nd. Varied magnetic behaviors were observed in these compounds, which are believed to be associated with the different interactions of Fe and rare earths.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The rare-earth orthoferrites have the general formula RFeO3, where R is a large rare-earth element ion, such as a rare-earth or Y ion. They crystallize in a distorted perovskite structure with an orthorhombic unit cell. The unit cell contains four equivalent iron ions, but the axes of the four surrounding octahedra are slightly tilted with respect to each other. RFeO3 is drawing considerable attention for its applications in various fields, such as fuel cells, catalysts, gas sensors, semiconductors, magnetic materials, and magnetic resonance imaging (MRI) in biomedicine [1–4]. The remarkable properties of orthoferrites, such as high domain-wall velocity and the existence of Bloch lines, have significance for applications in magnetic field sensors and magneto-optical data storage devices [5, 6]. Meanwhile, synthesis of nano-scaled materials is gaining tremendous interest, since ultrafine powder usually exhibits superior properties such as large surface area, higher chemical reactivity, and better sinterability [7].
This family is characterized with interesting magnetic properties. Its weak ferromagnetism was systematically investigated in Ref. [8]. In this work, the influence of 4f electrons of rare earth on the RFeO3 orthoferrites was initially investigated. The outside electron structure of Y3+, Nd3+ and La3+ is s2p6, however La, Nd and Y atoms belong to different groups of transition elements (4f and 3d respectively). As important rare earth ions, La3+ has no electrons in 4f, Nd3+ has 3 electrons in 4f. Thus, Magnetic measurements were performed on the prepared YFeO3, LaFeO3 and NdFeO3 nanopowder.
Traditional high temperature solid-state reaction has been used to prepare RFeO3, which requires rather high sintering temperature (up to 1600 °C) and suffers from incomplete reaction of the raw materials and easy formation of undesired phases (i.e., R3Fe5O12 which is more favored by growth kinetics studies) [9, 10]. Attempts to obtain RFeO3 ultrafine powders have recently been performed by the polyol, sol–gel, heterobimetallic precursor, sonochemical, hydrothermal and combustion methods, etc. [4, 7, 11–14]. Compared with these wet chemical methods, the sol–gel combustion synthesis of RFeO3 exhibits many advantages. The method uses inexpensive precursor, and the experimental process is more simple and easier to operate. The sol–gel combustion method involves a rapid exothermic redox reaction between the metal nitrates and an appropriate fuel, which is ignited at a temperature much lower than the actual phase formation temperature. Therefore, this approach is considered as an attractive method to obtain single phase nanopowder.
In this work, we report the synthesis of nano-crystalline RFeO3 powder by the sol–gel combustion method. In addition, the structure, morphology and magnetic properties of nano-crystalline RFeO3 were also investigated.
2 Experiments
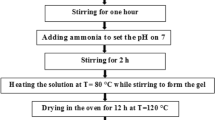
Rare earth oxides R2O3 (99.99% purity), Fe(NO3)3·9H2O(99.99% purity) and citric acid (AR) were used as raw materials. Firstly, the high-purity R2O3 and Fe(NO3)3·9H2O in the stoichiometric mole ratio of R:Fe = 1:1 were accurately weighted out, respectively. R2O3 was dissolved in nitric acid and diluted with distilled water. The concentration of the nitric acid is controlled at about 40–50%. Fe(NO3)3·9H2O was also dissolved in distilled water and adequately mixed with the rare earth nitrates. Citric acid was then added as a fuel to the above solution containing R3+ and Fe3+ to yield a citrate–nitrate ratio of 1.0. After mixing, the final solutions were heated and concentrated by slow evaporation at 85 °C under continuous stirring using a magnetic agitator for several hours. Then the yellowish transparent gels were obtained. Subsequently, the gel was ignited by increasing the temperature up to 250 °C. The dried gel burnt in a self-propagating combustion manner and large volume of brown fume evolved. Finally, a voluminous porous powder was obtained and the powder was calcined at different temperatures in air for 2 h. The experimental procedure is shown as Fig. 1.
Phase identification was checked by powder X-ray diffraction (XRD, D/Max-2550 V, Rigaku, Japan) using Cu Kα radiation and nickel as the filter. The average grain sizes of the powder were estimated following the Scherrer’s equation. The magnetic measurements of the samples were performed on a superconducting quantum interference device magnetometer (Quantum design, PPMS-9).
3 Results and discussion
3.1 Nanocrystalline powders synthesis
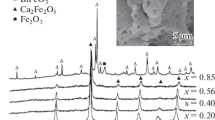
The rare-earth orthoferrites RFeO3 nanocrystalline powders, such as YFeO3, NdFeO3, LaFeO3 etc. were successfully synthesized with the sol–gel combustion method [15, 16], XRD measurement indicates that the synthesized powder is single phase of orthoferrite. The XRD patterns of RFeO3 are shown in Fig. 2. The crystallite size of the heat-treated powders was calculated according to the X-ray line-broadening method, using the Scherrer’s equation: D = 0.89λ/Bcosθ, where D is the crystallite size; λ is the X-ray wavelength (0.15405 nm for Cu Kα); B is the corrected FWHM of the diffraction peak; and θ corresponds to the diffraction angle. The average crystallite size was calculated to be about 30–40 and 60–90 nm for as-burnt powder. The rare-earth orthoferrites RFeO3 nanocrystalline powders can be obtained through calcining at 600–900 °C, the SEM morphology of the YFeO3 powder calcined at 800 °C is shown as Fig. 3.
Generally, the sol–gel combustion often uses fuel, such as citric acid, amino acid, carbamide and hydrazine compound. Amino acid and hydrazine compound is relatively expensive, and the chelate and complex ability of carbamide to metallic cations is weak. Moreover, the combustion temperature of carbamide is up to 1,600 °C, which is unhelpful to obtain ultrafine powder with good dispersion. Surprisingly, citric acid is a kind of polycarboxylic acid, having low price, strong chelate ability and moderate combustion temperature, which is a good choice as fuel for preparation of RFeO3. The sol–gel is sophisticated technique and requires stringent drying conditions and expensive alkoxide precursors [17]. What is more significant is that the calcination temperature for RFeO3 is rather low by this sol–gel combustion method, since the phase of RFeO3 can be directly formed in the as-burnt powder. Generally, the other methods in related references require calcination temperature up to 800 °C to form the desired ReFeO3 phase.
During the self-propagating combustion process, citric acid acts as the fuel for the combustion process and is oxidized by the nitrates, a large amount of brown fume evolving. The stoichiometric combustion reaction is:
3.2 Magnetic property
The elementary cell of RFeO3 orthoferrites consists of four formulae ABO3 with atoms distributed in positions such as the ones shown in Fig. 4. The structure can be visualized as a three-dimensional network of strings of FeO6 octahedra. The orthoferrite RFeO3 crystallizes in a distorted perovskite structure with an orthorhombic unit cell. The distortion from the ideal cubic perovskite is mainly in the position of the R ions, where the Fe3+ ions are present in an essentially octahedral environment. In orthoferrite RFeO3, the six-coordinated iron octahedra have been slightly tilted, lowering the symmetry to orthorhombic. The magnetic moments of iron and rare earth atoms as well as the interactions between them are the source of the magnetic properties of these orthoferrites. Rare earth and iron atoms belong to different groups of transition elements (4f and 3d respectively) with different magnetic moment values. It is common to divide all the interactions into the R–R, the Fe–Fe and the Fe–R ones, each one generally consisting of isotropic, antisymmetric and anisotropic symmetric super-exchanges. A well-known feature of orthoferrites is the strong anisotropy of various origins, which depends essentially on the rare-earth atoms of compounds. The antisymmetric component of the super-exchange interaction manifests itself as the non-colinear magnetic structures of various types which are characteristic for this family of compounds. The room-temperature magnetic hystereses of these compounds (YFeO3, LaFeO3 and NdFeO3) are shown in Fig. 5.
The magnetization versus magnetic field (M–H) curve of YFeO3 nanopowder displays obvious rectangular hysteretic loop, and the saturation magnetization (Ms) reaches 0.15 emu/g and coercive field Hc is about 40 kOe. Although the RFeO3 orthoferrites are canted antiferromagnets, YFeO3 exhibits ferromagnetic behavior, which maybe due to special outside ionic structure of Y3+ (3d0). In LaFeO3 nanopowder, there is a hysteresis loop at room temperature with a spontaneous magnetization. The shape of the hysteresis loop is also characteristic of weak ferromagnetism. However, even a field of 20 kOe does not saturate the magnetization curve, and the magnetization still has a tendency to increase, which is characteristic of the mainly anti-ferromagnetic ordering of the spins in the nanoparticles. The coercive field is about 137 Oe and the spontaneous magnetization is about 0.10 emu/g. The spontaneous magnetization is directly estimated by extrapolating the linear part of M(H) to H = 0 Oe [18]. The M–H curve of NdFeO3 nanopowder is also shown in Fig. 5, the maximum field applied is 20 kOe, too. Different from YFeO3 and LaFeO3, the shape of the hysteresis loop of NdFeO3 nanopowder exhibits typical characteristic of anti-ferromagnetism, the magnetization still has an identical tendency to increase even at a field of 20 kOe.
In addition, the saturation magnetization (Ms) of RFeO3 is no more than 0.30 emu/g in this work. It is obviously lower than those in the earlier references [19–21], in which the saturation magnetization of YFeO3, LaFeO3 and NdFeO3 are all more than 1 emu/g. However, the coercivity of RFeO3 in this work is similar with those in the earlier references. It maybe originated from the nanosized effect of RFeO3 powder because the average sizes in earlier references are all more than 200 nm. Nanocrystalline RFeO3 exhibits high coercivity and low saturation magnetization in reference [20].
The RFeO3 family shows various interesting magnetic coupling, due to different 4f electrons distribution of rare earth element and the interaction between Fe ions and rare earth element. Generally, the magnetic moments of iron and rare earth atoms as well as the interactions between them are the source of the magnetic properties of RFeO3. The magnetic superexchange interactions between the Fe ions result in collinear anti-ferromagnetic spin order which contributes to the anti-ferromagntic coupling along a particular crystallographic direction. However, low symmetry of the distorted orthorhombic perovskite-type structure leads to the tilt of FeO6 octahedra. Consequently, a canted spin order is also formed in the Fe lattice, which gives rise to weak ferromagnetism along another crystallographic direction [22, 23]. The FeO6 octahedra can be tilted to different degree depending on the size of the rare-earth cation, leading to different net magnetic moment [24].
As discussed above, typical ferromagnetic coupling is observed in YFeO3. However, the coercivity and remnant magnetization are small for LaFeO3 and NdFeO3. So their magnetic behaviors were investigated in more detail. The magnetization versus temperature M (T) in 100 Oe field with zero-field-cooled (ZFC) and field-cooled (FC) modes for LaFeO3 and NdFeO3 are shown in Fig. 6. NdFeO3 has two magnetic sublattices, and the Fe sublattice is antiferromagnetism, while the magnetic moments of Nd ions have negative temperature coefficient. Below 35 K, the magnitude of Nd moments is larger than that of Fe cations, and ferromagnetic-like situation which is revealed by ZFC curve as shown in Fig. 6a may occur. However, moments of Nd decreases with the increasing temperature, which induces the drop in magnetization up to 35 K. Thereafter, the antiferromagnetism of Fe sublattice becomes prominent [25]. The behavior of LaFeO3 is different from that NdFeO3. The magnetization in FC curve decreases continuously with increasing temperature, and a cusp in the ZFC curve is observed around 38 K. These characteristics are interpreted as the evidence of magnetic frustration or glassy behavior [26].
4 Conclusions
The polycrystalline nano-sized RFeO3 powders were synthesized by the gel combustion method, and the average crystallite size is 60–90 nm. Nanocrystalline RFeO3 exhibits high coercivity and low saturation magnetization due to the nanosized effect. As anti-ferromagntic materials, some RFeO3 orthoferrites exhibit weak ferromagnetism due to the empty of 4f electrons. Maybe the empty of 4f electrons in R3+ ions is also a main reason of anti-ferromagnetism in RFeO3 orthoferrites exhibiting weak ferromagnetism, which needs further investigation and research in the future.
References
Minh NQ (1993) J Am Ceram Soc 76:563
Niu XS, Du WM, Du WP (2004) Sens Actuators B 99:399
Fredrik S, Marc AF, Rodrigo PJ, Anna K, Teodor V, Maria E, Kajsa U, Per-Olov K (2008) Nanotechnology 19:085608
Delmastro A, Mazza D, Ronchetti S, Vallino M, Spinicci R, Brovetto P, Salis M (2001) Mater Sci Eng B 79:140
Ripka P, Vertesy G (2000) J Magn Magn Mater 215:795
Didosyan YS, Hauser H, Reider GA, Toriser W (2004) J Appl Phys 95:7339
Mathur S, Shen H, Lecerf N, Kjekshus A, Fjellvag H, Goya GF (2002) Adv Mater 14:1405
Bombik A, Lesniewska B, Pacyna AW (2000) J Magn Magn Mater 214:243
Ravindranathan P, Komarneni S, Roy R (1993) J Mater Sci Lett 12:369
Buscaglia V, Caracciolo F, Bottino C, Leoni M, Nanni P (1997) Acta Mater 451:213
Qi X, Zhou J, Yue Z, Gui Z, Li L (2003) Ceram Int 29:347
Zheng W, Liu R, Peng D, Meng G (2000) Mater Lett 43:19
Sivakumar M, Gedanken A, Bhattacharya D, Brukental Y, Yeshurun Y, Zhong W, Du Y, Felner I, Nowik I (2004) Chem Mater 16:3623
Rajendran M, Bhattacharya AK (2006) J Eur Ceram Soc 26:3675
Shen H, Cheng G, Wu A, Xu J, Zhao J (2009) Phys Stat Sol (a) 206:1420
Wu A, Cheng G, Shen H, Xu J, Chu Y, Ge Z (2009) Asia-Pac J Chem Eng 4:518
Vajargah SH, Hosseini HRM, Nemati ZA (2007) J Alloys Compd 339:430
Bozorth RM, Williams HJ, Walsh DE (1956) Phys Rev 103:572
Mathur S, Veith M, Rapalaviciute R, Shen H, Goya GF, Filho WL, Berquo TS (2004) Chem Mater 16:3623
Qi X, Zhou J, Yue Z, Gui Z, Li L (2002) Mater Chem Phys 78:25
Slawinski W, Przenioslo R, Sosnowska I, Suard E (2005) J Phys Condens Matter 174:605
Parida SC, Rakshit SK, Singh Z (2008) J. Solid State Chem 181:101
Mathur S, Veith M, Rapalaviciute R, Shen H, Goya GF, Filho WL, Berquo TS (2004) Chem Mater 16:1906
Cristobal AA, Botta PM, Bercoff PG (2009) Mater Res Bull 44:1036
Zhang Y, Zhang H, Yin J, Zhang H, Chen J, Wang W, Wu G (2010) J Magn Magn Mater 322:2251
Mao J, Sui Y, Wang X, Yang Y, Zhang X, Wang Y, Wang Y, Liu W (2008) J Alloy Compd 509:4950
Acknowledgments
The authors would like to acknowledge the financial support from National Natural Science Foundations of China (Grant No. 50932003 and 51002097). This work is also supported by the Opening Project of State Key laboratory of Crystal Material (Grant No. KF1006).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, A., Shen, H., Xu, J. et al. Preparation and magnetic properties of RFeO3 nanocrystalline powders. J Sol-Gel Sci Technol 59, 158–163 (2011). https://doi.org/10.1007/s10971-011-2474-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-011-2474-z