Abstract
Well-aligned ZnO nanorods are obtained by a liquid phase epitaxial growth on the indium-doped tin oxide glass deposited with a ZnO thin film as the seed layer, which is prepared by combining a sol–gel process and a spin coating technique. The effects of water content in the sol and heat treatment temperature on the properties of the ZnO thin film are investigated. Relationship among the seed layer, the growing time, the growing temperature, the concentration of Zn2+ in the solution, the anions in the solution and the resulting ZnO nanorods are discussed in detail. X-ray diffraction analysis and scanning electronic microscopy are employed to characterize the structural and morphological properties of the resulting ZnO nanorods. Results indicate that the ZnO nanorods with a preferred orientation show a single crystal with a wurtzite structure in the direction of [0001], the diameter of the ZnO nanorods seems to depend on the size of the seed grain, while the length of the ZnO nanorods is determined by the growing time and the growing temperature.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Semiconductor ZnO has attracted more and more attentions in recent years because it can lead to lasing action based on exciton recombination even at room temperature. During the past several decades, ZnO with different structures such as powder, films and porous diaphragm have been widely studied in detail, which leads to the discovery of many new properties of the semiconductor. Recently, there has been a growing interest in ZnO nanorods for their high surface area and specific crystalline orientation [1] and broad applications in many fields such as solar cells [2], photodetectors [3], light-emitting diodes [4], ultraviolet lasers [5], optical modulator wave guides [6], and gas sensors, etc. [7]. Therefore, various fabrication techniques have been employed to grow ZnO nanorods. For example, Ref. [5] reported a vapor-liquid-solid growth method, which uses an Au thin film as the catalysts for the growth of the nanowires and the diameter of the grown nanowires can be controlled by the sizes of the Au nanocluster. Ref. [8] reported a synthesis of highly ordered and narrow dispersive ZnO nanowires whose diameter and length can be controlled by the anodic alumina membranes (AAM) template. In addition, some other methods, including electrochemical deposition [9–11], chemical vapor deposition [12], and catalyst-based chemical vapor deposition [13], have been also employed to synthesize ZnO nanorods with a controlled morphology. However, these mentioned methods are not simple enough or need expensive equipments, which are not suitable for commercial run to fabricate the ZnO nanorods for various applications. Although ZnO nanorods derived from liquid growths have been also reported in Refs. [14, 15], the details about the growing process are still unclear.
Here, we report a simple process to fabricate well-aligned ZnO nanorods on the indium tin oxide (ITO) glass through liquid phase epitaxy process from the mixture of zinc nitrate and sodium hydroxide as precursory solution. Before the epitaxy process, a highly preferred orientation ZnO thin film is first prepared on the ITO glass by combining a simple and low-cost sol–gel process and a spin-coating technique. The effect of addition content of water in the sol on the spin-coating process was studied. The optimum temperature for obtaining the preferred orientation film along (002) plane is proved to be at 500 °C. The resulting nanorods have single crystals with a terminal of (002) plane, which is in parallel with the glass substrate. The relationship between the morphology and the growing conditions is discussed in detail. Results indicate that the seed layer plays a crucial role in the liquid epitaxy process of the ZnO nanorods. The diameter of the ZnO nanorods seems to depend on the property of the seed layer, rather than the growing time and temperature in the initial period of the growing. On the contrary, the length of the nanorods is determined by the growing time, the growing temperature and the concentration of Zn2+ in the solution. According to above these facts, a growth mechanism is proposed to explain the growing process of the ZnO nanorods as follows: The ZnO nanorod starts to grow along (002) plane when the seed layer is immersed into the supersaturate solution of Zn ion, this should be attributed to the fact that the (002) plane has the highest surface energy and growing velocity, as well as the intense induction and space constraint of the dense seed layer. It should be mentioned here that the most significant merit for our method is cost-effective, simple, and suitable for mass production of the ZnO nanorods.
2 Experimental
2.1 Materials
All the chemicals used in our experiment are analytical pure and are purchased from Sinopharm Group Chemical Reagent Co. Ltd. China.
2.2 Preparation of ZnO seed layer
ZnO thin film, which is used as seed layer for epitaxial growth of the ZnO nanorod, was prepared by combining a sol–gel process and a spin-coating technique. The solution for ZnO thin film layer was prepared by following the steps as reported in Ref. [16]. It should be stressed here that as compared to Ref. [16], the only difference is that in order to investigate the effect of water content in the solution on the property of the resulted ZnO thin film, three kinds of sols with different water contents were prepared, which include that molar ratios of monoethanolamine (MEA): zinc acetate: deionized water are: A = 1:1:0, B = 1:1:2, and C = 1:1:4. Finally, the mixed solution was stirred for about 30 min at a temperature of 60 °C and then the mixed sols were aged at room temperature for another 24 h. Prior to the spin-coating process, the substrates including Si wafer and ITO glass were ultrasonically cleaned in acetone and ethanol, respectively. The resultant sol was employed to fabricate the thin film by the spin-coating process and the film-coated samples were immediately put in a furnace at 200 °C and 300 °C for about 5 min and 10 min, respectively. Then the films were further heated at different temperature from 400 to 750 °C for 1 h. In order to study the relationship between the seed layer and the morphology of the ZnO nanorods, the ZnO thin films with different thickness were prepared by multi-spin-coating process.
2.3 Liquid epitaxial growth of ZnO nanorods
The precursory solution for epitaxial growth was prepared from zinc nitrate hexahydrate and sodium hydroxide. The molar ratio of Zn2+ to OH− is 1:20 and the concentration of sodium is 0.8 M. The ITO substrates with and without seed layers were immersed into the precursory solution perpendicularly at different growth temperature and time. In order to investigate the effect of the concentration of Zn2+ in the solution, the precursory solutions with different Zn2+ concentrations were prepared to grow ZnO nanorods. Furthermore, zinc nitrate was replaced by zinc chloride and zinc acetate, responsively, so as to investigate the effects of the anions on the morphological properties of the ZnO nanorods.
2.4 Characterization
X-ray diffraction (XRD) analysis was employed to characterize the orientation and crystallinity of the ZnO seed layer and the resultant ZnO nanorods, using a D/max 2400 X Series X-ray diffractometer. The X-ray radiation source used was Cu Kα, obtained at 40 kV, 100 mA and the scanning speed was 10o min−1 at a step of 0.02o. A field emission scanning electron microscopy (JSM-6700F, JEOL Inc., Japan) was used to characterize the morphology of the ZnO seed layer and the ZnO nanorods. The UV–Vis absorption spectrum of the ZnO thin film was obtained from the thin film deposited on ITO glass in the range of 250–800 nm by a JASCO V-570 UV/VIS/NIR Spectrometer.
3 Results and discussion
It was noted that the solution C with an addition of much water content became opacity seriously after aging 24 h in air, indicating that it is not suitable for fabricating the ZnO thin film. That is to say, the amount of water added into the sol is too much to keep the sol stable at a relative long time. However, the solutions A and B are still transparent and thus they were used to fabricate the seed layer on the ITO glass by the spin-coating technique. It is found that some macroscopic pore-like structures can be clearly observed on the surface of all the film-coated samples if without drying, and it is also noted that the density of the macroscopic pore-like structure seems to be related to the humidity of environmental atmosphere. However, the number of these pore-like structures in the thin film prepared from the solution B is always less than that of the thin film prepared from solution A. In addition to, few pore-like structures was found if the thin films were dried immediately after being spin-coated. One possible explanation for this phenomenon is that the occurrence of these pore-like structures is probably caused by the non-homogeneous coactions between the gels and water vapor in the environmental atmosphere. Since some additional water is added and thus the Zn ions hydrolyze more adequately as compared to solution A, it is probably that the thin film prepared from solution B suffers less from this coactions, which results in much less pore-like structures. Whereas the detail reason for this phenomenon is still unknown at present stage. However, it should be stressed here that the pore-like structures can be avoided by drying the samples immediately after the spin-coating.
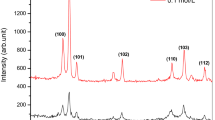
Figure 1 shows the XRD patterns of the ZnO thin films obtained by different post-heat treatment temperatures for 1 h. It can be seen from those samples heated below 550 °C that only the peak at (002) can be observed and the relative intensity of this peak increases obviously with an increase of the heat treatment temperature from 400 to 500 °C, and reaches its highest point when the post-heat treatment temperature is increased to 500 °C. However, with further increase the post-heat treatment temperature to 550 °C or above, besides the diffraction peek at (002), some other peaks at (100) and (101) can be also clearly observed and the relative intensity of the peek at (002) starts to decrease. These results indicate that the optimum post-heat treatment temperature to obtain highly preferred orientation ZnO thin films along (002) plane is at 500 °C and the ZnO thin film obtained at present conditions shows a wurtzite crystal structure. A highly preferred orientation ZnO thin films along (002) plane can be obtained under present processing conditions. A possible explanation is as follows: The crystallization process can be considered as an equilibrium process of the atoms’ disordered thermal motion and ordered crystallization. Although the crystallization of the thin film has a trend of along (002) plane, the crystallization process at the beginning of the heat treatment is random. With extension of the heat treatment time and increase of the heat treatment temperature, atoms with enough energy can get across the crystal boundary and the random crystallization becomes preferred orientation, provided that the heat treatment temperature is at a proper value, until arrive at a balance process where all the crystal grains grow along c axis. However, this balance can not be arrived if either the heat treatment temperature is below or above the certain proper value, since the energy of the atoms’ thermal motion is either too low to get across the crystal boundary or too high to maintain the balance. So there is an optimum temperature to maintain this balance and obtain the highly preferred orientation thin film.
The morphologies of the ZnO thin films obtained by multi-spin-coating process are shown in Fig. 2. Figure 2a shows a one-layer thin film and has a film thickness of 55 nm, and the thickness of the thin films in Fig. 2b and c is 120 nm and 160 nm, respectively. It can be observed from the insert of Fig. 2 that the average size of the seed grain increases gradually with the increase of the thin film thickness and reaches maximum as shown in Fig. 2c, which is still less than 50 nm. Furthermore, the morphology of the seed grain becomes clearer with the increase of the thin film thickness. Figure 3 shows the UV–Vis absorption spectrum of the as-prepared ZnO thin film deposited on ITO glass by a single spin-coating process. It can be seen that the ZnO thin film has a strong absorption in the UV area, but the absorption in visible area is weak. The absorption edge is about 375 nm, corresponding to a band gap of 3.31 eV, which is very close to the intrinsic band-gap.
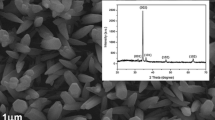
Well-aligned ZnO nanorods were obtained on the ITO glass by the liquid phase epitaxial growth process in a time range from 0.5 to 4 h. It was noted that the nanorods with a length of about 1.0 μm and an average diameter of about 40 nm can be easily obtained after a growing period for 0.5 h at 80 °C. Figure 4 shows XRD pattern of the ZnO nanorod grown in precursory solution at 80 °C for 1 h. There is only an intense peak at (002) to have been clearly observed, indicating that the grown ZnO nanorods are also single crystals structure with a terminal along (002) plane of the seed layer, which parallels with the ITO glass substrate. That is to say, the highly preferred orientation ZnO nanorods can be easily obtained under present process conditions. Figure 5 shows the SEM images of the ZnO nanorods grown on ITO at a temperature of 80 °C for different time. Figure 5a and b presents the top view and cross-sectional view of the ZnO nanorods grown for 1 h, and Fig. 5c and d shows the top view and cross-sectional view of the ZnO nanorods grown for 4 h, respectively. Results from Fig. 5 indicate that well-aligned and faceted ZnO nanorods with narrow size distribution in diameter can be easily obtained, especially, the ZnO nanorods from Fig. 5c shows a perfect hexagonal shape at (002) plane. The length of the ZnO nanorods as shown in Fig. 5b and d is about 2.8 and 3.7 μm, respectively. More detailed experimental results indicate that the ZnO nanorods have the highest growth rate along the c axis in the initial 1 h of growing, but its length seems to remain little change even the growing time is increased beyond 3 h. On the contrary, the lateral growth velocity is much lower than the longitudinal growth velocity, so that the diameter of the nanorods appears to be independent on the growing time, provided that the growing time is less than 1 h. However, if the growing time is further prolonged, the adjacent nanorods may coalesce together and result in some thicker nanorods, which may be the reason of initiation of fortuitous section ZnO nanorods.
The ZnO nanorods grown under different temperatures were also investigated. The results indicate that the growth along c axis depends on the growing temperature obviously. For example, our result indicates that there is no ZnO nanorod growth to have been observed at the temperature of 40 °C or below. The nanorods grown at 65 °C for 1 h show an average diameter of 90 nm and an average length of 1.7 μm, and these values are turned to 100 nm and 2.8 μm when the growing temperature is increased to 80 °C. Actually, the diameter of the nanorods only varies a little in the range from 40 to 95 °C in our experiment, which indicates that the growth velocity of (002) plane is much more sensitive than that of those lateral planes. In addition to, the ZnO nanorods grown on seed layers with different thickness, which have crystal grains of different sizes, were also studied in our experiment. Figure 6 shows the morphologies of the ZnO nanorods grown from those seed layers with different thickness. Generally speaking, the thicker the thickness of the seed layer is (crystal grain is bigger), the thicker the nanorods grow. These indicate that the diameter of the nanorods is determined by the size of the seed grain at the initial growing period to some extent. In order to study the relationship between the seed layer and the obtained ZnO nanorods, ITO substrate without deposited seed layer was employed to grow ZnO nanorods. Result indicates that no any nanorods were observed on the substrate, which agrees with that as reported in Ref. [15]. This suggests that the seed layer is a critical issue for the growth of the ZnO nanorods in liquid growth process. To explore the effect of the seed layer on the ZnO nanorods more clearly, the growing time was shortened to 0 (just heated the water from room temperature to 80 °C) and 5 min, respectively, and Figs. 6 (d) and (e) show the SEM images of the resultant nanorods from the seed layer prepared by three times spin-coating process. As compared with the SEM image of the corresponding seed layer as shown in Fig. 2 (c), it can be seen that during the first few minutes, the nanorods have an average diameter almost the same as the seeds, which can be attributed to the epitaxy growth along the surface of the seeds. However, when the growing time was prolonged to 30 min, the diameter of the nanorods as shown in Fig. 6 (c) becomes larger than that of the seeds, indicating the growth along lateral. According to Ref. [14], this phenomenon results from the strain relaxation of the seeds. It should be noted that the lateral growth rate in our experiment is not as high as that as reported in Ref. [14]. Such difference may be related to the different fabrication process of the seed layer, which results in different stress in the seeds. It can be concluded based on above these results that the properties of the seed layer are of great importance for the growing process of the nanorods.
In order to investigate the effect of the concentration of Zn2+ in the solution on the morphological properties of the obtained nanorods, two kinds of precursory solutions with a Zn2+ concentration of 0.02 M and 0.06 M, respectively, were synthesized to grow ZnO nanorods. Results show that there are no any nanorods to have been observed on the surface of the seed layer immersed in the precursory solution with a Zn2+ concentration of 0.02 M after 1 h at 80 °C. Figure 7a shows a SEM image of the sample obtained in solution with a concentration of 0.06 M. It can be observed that the adjacent nanorods coalesce together, which looks like a ZnO film rather than a ZnO nanorod. It indicates that it is very difficult to obtain highly-ordered nanorods from the solution with a Zn2+ concentration of 0.06. A possible explanation for these phenomena is as follows: in the solution with a Zn2+ concentration of 0.02 M, there are so few Zn2+ ions (or Zn(OH) 2−4 ) that their thermal motions are not able to break the balance of the solution, thus no nucleus of ZnO crystal can form, resulting in that the growth of the nanorods is impossible. On the contrary, when the concentration of Zn2+ is increased to 0.06 M, the balance of the solution is destroyed totally at the very beginning of the growing process. That is to say, so many nucleuses are formed around the seed layer that the epitaxy growing along (002) phase of the ZnO crystal is disturbed seriously, which leads to the adjacent nanorods growth together. These results indicate that the concentration of Zn2+ in the precursory solution has an important impact on the morphology of the resultant ZnO nanorods. Further, zinc chloride and zinc acetate were also chosen as the substitutes of zinc nitrate to study the effect of the anions in the solution on the morphology of the nanorods. Figure 7b shows the image of the nanorods grown in precursory solution prepared by zinc chloride at a growth temperature of 80 °C for 1 h. The nanorods are about 4.5 μm in length, which is much longer than those grown in precursory solution prepared by zinc nitrate at the same conditions. However, there is no much difference in the average diameter between them. The similar results were also found in those nanorods grown from zinc acetate precursory solution. It should be mentioned here that the growth of the nanorods is also impossible from the precursory solution prepared either by zinc chloride or by zinc acetate, provided that the concentration of Zn2+ is decreased to 0.02 M, which further demonstrates that the concentration of Zn2+ is a critical issue in the liquid growing process of the ZnO nanorods. It can be concluded based on above these results that the anion in the solution can affect the growth process of the ZnO nanorods seriously, though the detailed reason for this phenomenon is unknown now and further study is being done in our laborary.
It should be mentioned here that the pH value of the precursory solution employed in our experiment is between 13.15 and 13.66 and the form of zinc ion is Zn(OH) 2−4 predominantly. The Zn(OH) 2−4 ion can exist stably in the solution at room temperature, which should be attributed to the Coulomb force and hydration sphere surround it [17]. However, with an increase of the growing temperature, these ions’ thermal motion becomes more drastic and makes them easer for the ions to react each other. Since (002) plane is formed by Zn atoms with a –OH terminal [18], the Zn(OH) 2−4 ion is more likely to react at the interface between the ion and the solution. In summary, chemical reactions may occur at the interface between the crystal and the solution as follows [19, 20]
As reported that the growth velocity of ZnO crystal with wurtzite structure under hydrothermal conditions is \( V_{(0001)} > V_{(0110)} > V_{(1000)} \) [21]. In addition, it is also possible that the induction and the space constraint of the dense seed layer make the epitaxial growth along lateral much more difficult. These indicate that the growth process of the ZnO nanorods may be an epitaxy along (002) plane of the seed layer due to above–mentioned these reasons, but if the growing time is long enough, those adjacent nanorods may coalesce together and result in thicker and fortuitous section ZnO nanorods.
4 Conclusions
ZnO nanorods have been successfully grown on ITO glass by the liquid epitaxy process. XRD results indicate that the optimum heat treatment temperature for a preferred orientation seed film along (002) plane is 500 °C, and the resulting nanorods have a single crystal structure with a terminal plane of (002). Effects of the seed layer, the growing time, the growing temperature, the concentration of Zn2+ in the solution and the anions in the solution on the resulting ZnO nanorods have been also investigated. Results indicate that the length of the resulting nanorods depends on the growing time and the growing temperature highly. However, the diameter of the nanorods seems to be determined by the size of the seed grain at the initial growing period. The probable reason of this phenomenon is that the (002) plane has the highest surface energy and the growing velocity, as well as due to the induction and the space constraint of the dense seed layer.
References
Vayssieres L, Keis K, Lindquist SE, Hagfeldt A (2001) J Phys Chem 105:3305–3352
Law M, Greene LE, Johnson JC, Saykally R, Yang PD (2005) Nat Mater 4:455–459
Liang S, Sheng H, Liu Y, Hiu Z, Lu Y, Shen H (2001) J Cryst Growth 225:110–113
Saito N, Haneda H, Sekiguchi T, Ohashi N, Sakaguchi I, Koumoto K (2002) Adv Mater 14:418–421
Huang MH, Mao S, Feick H, Yan HQ, Wu YY, Kind H, Weber E, Russo R, Yang PD (2001) Science 292:1897–1899
Koch MH, Timbrell PY, Lamb RN (1995) Semicond Sci Tech 10:1523–1527
Golego N, Studenikin SA, Cocivera M (2000) J Electrochem Soc 147:1592–1594
Li Y, Meng GW, Zhang LD, Phillipp F (2000) Appl Phys Lett 76:2011–2013
Konenkamp R, Boedecker K, Lux-Steiner MC, Poschenrieder M, Zenia F, Levy-Clement C, Wagner S (2000) Appl Phys Lett 77:2575–2577
Izaki M, Omi T (1996) Appl Phys Lett 68:2439–2440
Pauporte T, Lincot D (1999) Appl Phys Lett 75:3817–3819
Park WI, Kim DH, Jung SW, Yi GC (2002) Appl Phys Lett 80:4232–4234
Liu DF, Xiang YJ, Liao Q, Zhang JP, Wu XC, Zhang ZX, Liu LF, Ma WJ, Shen J, Zhou WY, Xie SS (2007) Nanotechnology 18(405303):1–5
Wu WB, Hu GD, Cui SG, Zhou Y, Wu HT (2008) Cryst Growth Des 8:4014–4020
Zhao J, Jin ZG, Liu XX, Liu ZF (2006) Jour Ceram Soc 26:3745–3752
Ohyama M, Kozuka H, Yoko T (1997) Thin Solid Films 306:78–85
Peterson RB, Fields CL, Gregg BA (2004) Langmuir 822:85–90
Li WJ, Shi EW, Zhong WZ, Yin ZW (1999) J Cryst Growth 203:186–196
Laudise RA, Kolb ED (1963) Am. Mineral 48:642–648
Suscavage M, Harris M, Bliss D, Yip P, Wang SQ, Schwall D, Bouthillette L, Bailey J, Callahan M, Look DC, Reynolds DC, Jones RL, Litton CW (1999) MRS Internet J Nitride Semicond Res 4S1, G3. 40
Laudise RA, Ballman AA (1960) J Phys Chem 64:688–691
Acknowledgments
This work was supported by the Ministry of Science and Technology of China through 863-project under grant 2009AA03Z218, the Science and Technology Developing Project of Shaanxi Province (2008K01-34), and Xi’an Applied Materials Innovation Fund (XA-AM-200805).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yin, Y.T., Que, W.X. & Kam, C.H. ZnO nanorods on ZnO seed layer derived by sol–gel process. J Sol-Gel Sci Technol 53, 605–612 (2010). https://doi.org/10.1007/s10971-009-2138-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-009-2138-4